rhEPO (recombinant human eosinophil peroxidase): expression in Pichia pastoris and biochemical characterization
- PMID: 16396635
- PMCID: PMC1422775
- DOI: 10.1042/BJ20051385
rhEPO (recombinant human eosinophil peroxidase): expression in Pichia pastoris and biochemical characterization
Abstract
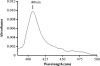
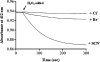
A Pichia pastoris expression system has for the first time been successfully developed to produce rhEPO (recombinant human eosinophil peroxidase). The full-length rhEPO coding sequence was cloned into the pPIC9 vector in frame with the yeast alpha-Factor secretion signal under the transcriptional control of the AOX (acyl-CoA oxidase) promoter, and transformed into P. pastoris strain GS115. Evidence for the production of rhEPO by P. pastoris as a glycosylated dimer precursor of approx. 80 kDa was determined by SDS/PAGE and gel filtration chromatography. Recombinant hEPO undergoes proteolytic processing, similar to that in the native host, to generate two chains of approx. 50 and 20 kDa. A preliminary biochemical characterization of purified rhEPO demonstrated that the spectral and kinetic properties of the recombinant wild-type EPO are comparable with those of the native enzyme and are accompanied by oxidizing activity towards several physiological anionic substrates such as SCN-, Br- and Cl-. On the basis of the estimated K(m) and kcat values it is evident that the pseudohalide SCN- is the most specific substrate for rhEPO, consistent with the catalytic properties of other mammalian EPOs purified from blood.
Figures



References
-
- Gleich G. J. Mechanisms of eosinophil-associated inflammation. J. Allergy Clin. Immunol. 2000;105:651–663. - PubMed
-
- Adamko D., Odemuyiwa S. O., Moqbel R. The eosinophil as a therapeutic target in asthma: beginning of the end, or end of the beginning? Curr. Opin. Pharmacol. 2003;3:227–232. - PubMed
-
- Bruynzeel-Koomen C. A., Van Wichen D. F., Spry C. J., Venge P., Bruynzeel P. L. Active participation of eosinophils in patch test reactions to inhalant allergens in patients with atopic dermatitis. Br. J. Dermatol. 1988;118:229–238. - PubMed
-
- Bousquet J., Chanez P., Vignola A. M., Lacoste J. Y., Michel F. B. Eosinophil inflammation in asthma. Am. J. Respir. Crit. Care Med. 1994;150:33–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

