Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress
- PMID: 16396985
- PMCID: PMC1815381
- DOI: 10.1210/en.2005-1041
Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress
Abstract
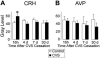
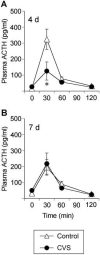
Chronic stress induces both functional and structural adaptations within the hypothalamo-pituitary-adrenocortical (HPA) axis, suggestive of long-term alterations in neuroendocrine reactivity to subsequent stressors. We hypothesized that prior chronic stress would produce persistent enhancement of HPA axis reactivity to novel stressors. Adult male rats were exposed to chronic variable stress (CVS) for 1 wk and allowed to recover. Plasma ACTH and corticosterone levels were measured in control or CVS rats exposed to novel psychogenic (novel environment or restraint) or systemic (hypoxia) stressors at 16 h, 4 d, 7 d, or 30 d after CVS cessation. Plasma ACTH and corticosterone responses to psychogenic stressors were attenuated at 4 d (novel environment and restraint) and 7 d (novel environment only) recovery from CVS, whereas hormonal responses to the systemic stressor were largely unaffected by CVS. CRH mRNA expression was up-regulated in the paraventricular nucleus of the hypothalamus (PVN) at 16 h after cessation of CVS, but no other alterations in PVN CRH or arginine vasopressin mRNA expression were observed. Thus, in contrast to our hypothesis, reductions of HPA axis sensitivity to psychogenic stressors manifested at delayed recovery time points after CVS. The capacity of the HPA axis to respond to a systemic stressor appeared largely intact during recovery from CVS. These data suggest that chronic stress selectively targets brain circuits responsible for integration of psychogenic stimuli, resulting in decreased HPA axis responsiveness, possibly mediated in part by transitory alterations in PVN CRH expression.
Figures






Similar articles
-
Chronic variable stress alters hypothalamic-pituitary-adrenal axis function in the female mouse.Physiol Behav. 2019 Oct 1;209:112613. doi: 10.1016/j.physbeh.2019.112613. Epub 2019 Jul 9. Physiol Behav. 2019. PMID: 31299374 Free PMC article.
-
The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress.Endocrinology. 2008 Feb;149(2):818-26. doi: 10.1210/en.2007-0883. Epub 2007 Nov 26. Endocrinology. 2008. PMID: 18039788 Free PMC article.
-
Effect of repeated lipopolysaccharide administration on tissue cytokine expression and hypothalamic-pituitary-adrenal axis activity in rats.J Neuroendocrinol. 2001 Aug;13(8):711-23. doi: 10.1046/j.1365-2826.2001.00684.x. J Neuroendocrinol. 2001. PMID: 11489088
-
Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression.Life Sci. 1998;62(22):1985-98. doi: 10.1016/s0024-3205(98)00027-7. Life Sci. 1998. PMID: 9627097 Review.
-
A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity.J Neuroendocrinol. 2009 Mar;21(4):351-8. doi: 10.1111/j.1365-2826.2009.01840.x. J Neuroendocrinol. 2009. PMID: 19207807 Free PMC article. Review.
Cited by
-
Psychoneuroendocrine alterations during 5 days of head-down tilt bed rest and artificial gravity interventions.Eur J Appl Physiol. 2013 Aug;113(8):2057-65. doi: 10.1007/s00421-013-2640-9. Epub 2013 Apr 12. Eur J Appl Physiol. 2013. PMID: 23579361
-
Critical features of acute stress-induced cross-sensitization identified through the hypothalamic-pituitary-adrenal axis output.Sci Rep. 2016 Aug 11;6:31244. doi: 10.1038/srep31244. Sci Rep. 2016. PMID: 27511270 Free PMC article.
-
Experimental gastritis leads to anxiety- and depression-like behaviors in female but not male rats.Behav Brain Funct. 2013 Dec 17;9:46. doi: 10.1186/1744-9081-9-46. Behav Brain Funct. 2013. PMID: 24345032 Free PMC article.
-
Chronic variable stress alters hypothalamic-pituitary-adrenal axis function in the female mouse.Physiol Behav. 2019 Oct 1;209:112613. doi: 10.1016/j.physbeh.2019.112613. Epub 2019 Jul 9. Physiol Behav. 2019. PMID: 31299374 Free PMC article.
-
Decreased glucose tolerance and plasma adiponectin:resistin ratio in a mouse model of post-traumatic stress disorder.Diabetologia. 2011 Apr;54(4):900-9. doi: 10.1007/s00125-010-2019-y. Epub 2010 Dec 22. Diabetologia. 2011. PMID: 21181395
References
-
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. - PubMed
-
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. - PubMed
-
- Armario A, Lopez-Calderon A, Jolin T, Balasch J. Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neurosci Biobehav Rev. 1986;10:245–250. - PubMed
-
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. - PubMed
-
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393:244–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

