Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease
- PMID: 16397132
- PMCID: PMC1895761
- DOI: 10.1182/blood-2005-09-3556
Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease
Abstract
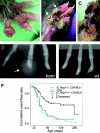
Macrophage actin-associated tyrosine phosphorylated protein (MAYP)/PSTPIP2, a PCH protein, is involved in the regulation of macrophage motility. Mutations in a closely related gene, PSTPIP1/CD2BP1, cause a dominantly inherited autoinflammatory disorder known as PAPA syndrome. A mutant mouse obtained by chemical mutagenesis exhibited an autoinflammatory disorder characterized by macrophage infiltration and inflammation, leading to osteolysis and necrosis in paws and necrosis of ears. Positional cloning of this recessive mutation, termed Lupo, identified a T to A nucleotide exchange leading to an amino acid substitution (I282N) in the sequence of MAYP. Mayp(Lp/Lp) disease was transferable by bone marrow transplantation and developed in the absence of lymphocytes. Consistent with the involvement of macrophages, lesion development could be prevented by the administration of clodronate liposomes. MAYP is expressed in monocytes/macrophages and in a Mac1+ subfraction of granulocytes. LPS stimulation increases its expression in macrophages. Because of the instability of the mutant protein, MAYP expression is reduced 3-fold in Mayp(Lp/Lp) macrophages and, on LPS stimulation, does not rise above the level of unstimulated wild-type (WT) cells. Mayp(Lp/Lp) mice expressed elevated circulating levels of several cytokines, including MCP-1; their macrophages exhibited altered cytokine production in vitro. These studies suggest that MAYP plays an anti-inflammatory role in macrophages.
Figures




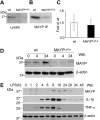

 ) and MaypLp/Lp (□) BMM. (B) Cytokine release in WT (
) and MaypLp/Lp (□) BMM. (B) Cytokine release in WT ( ) and MaypLp/Lp (□) BMMs stimulated with LPS for 24 hours. Duplicate measurements for BMMs from 2 mice of each genotype. Differences between WT and MaypLp/Lp are significant (P < .05; Student t test; n = 4). No significant differences were seen for the other 34 cytokines tested and listed in “Materials and methods.”
) and MaypLp/Lp (□) BMMs stimulated with LPS for 24 hours. Duplicate measurements for BMMs from 2 mice of each genotype. Differences between WT and MaypLp/Lp are significant (P < .05; Student t test; n = 4). No significant differences were seen for the other 34 cytokines tested and listed in “Materials and methods.”References
-
- Galon J, Aksentijevich I, McDermott MF, O'Shea JJ, Kastner DL. TNFRSF1A mutations and autoinflammatory syndromes. Curr Opin Immunol. 2000;12: 479-486. - PubMed
-
- McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97: 133-144. - PubMed
-
- Hull KM, Drewe E, Aksentijevich I, et al. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore). 2002;81: 349-368. - PubMed
-
- McDermott MF. A common pathway in periodic fever syndromes. Trends Immunol. 2004;25: 457-460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

