Lentiviral-mediated delivery of siRNAs for antiviral therapy
- PMID: 16397511
- PMCID: PMC7091755
- DOI: 10.1038/sj.gt.3302688
Lentiviral-mediated delivery of siRNAs for antiviral therapy
Abstract
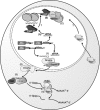
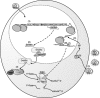
Lentiviral vectors portend a promising system to deliver antiviral genes for treating viral infections such as HIV-1 as they are capable of stably transducing both dividing and nondividing cells. Recently, small interfering RNAs (siRNAs) have been shown to be quite efficacious in silencing target genes. RNA interference is a natural mechanism, conserved in nature from Yeast to Humans, by which siRNAs operate to specifically and potently down regulate the expression of a target gene either transcriptionally (targeted to DNA) or post-transcriptionally (targeted to mRNA). The specificity and relative simplicity of siRNA design insinuate that siRNAs will prove to be favorable therapeutic agents. Since siRNAs are a small nucleic acid reagents, they are unlikely to elicit an immune response and genes encoding these siRNAs can be easily manipulated and delivered by lentiviral vectors to target cells. As such, lentiviral vectors expressing siRNAs represent a potential therapeutic approach for the treatment of viral infections such as HIV-1. This review will focus on the development, lentiviral based delivery, and the potential therapeutic use of siRNAs in treating viral infections.
Gene Therapy (2006) 13, 553-558. doi:10.1038/sj.gt.3302688; published online 5 January 2006.
Figures
Similar articles
-
Lentivirus-mediated RNA interference therapy for human immunodeficiency virus type 1 infection.Hum Gene Ther. 2006 May;17(5):479-86. doi: 10.1089/hum.2006.17.479. Hum Gene Ther. 2006. PMID: 16716105 Review.
-
Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection.Curr HIV Res. 2004 Apr;2(2):185-91. doi: 10.2174/1570162043484906. Curr HIV Res. 2004. PMID: 15078182 Review.
-
Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1.Gene Ther. 2005 Jul;12(14):1133-44. doi: 10.1038/sj.gt.3302509. Gene Ther. 2005. PMID: 15750613
-
Inhibition of HIV-1 infection by lentiviral vectors expressing Pol III-promoted anti-HIV RNAs.Mol Ther. 2003 Aug;8(2):196-206. doi: 10.1016/s1525-0016(03)00165-5. Mol Ther. 2003. PMID: 12907142
-
Lentiviral vector delivery of siRNA and shRNA encoding genes into cultured and primary hematopoietic cells.Methods Mol Biol. 2008;433:287-299. doi: 10.1007/978-1-59745-237-3_18. Methods Mol Biol. 2008. PMID: 18679631
Cited by
-
The Dual Role of AQP4 in Cytotoxic and Vasogenic Edema Following Spinal Cord Contusion and Its Possible Association With Energy Metabolism via COX5A.Front Neurosci. 2019 Jun 14;13:584. doi: 10.3389/fnins.2019.00584. eCollection 2019. Front Neurosci. 2019. PMID: 31258460 Free PMC article.
-
Recombinant lentivirus-delivered short hairpin RNAs targeted to conserved coxsackievirus sequences protect against viral myocarditis and improve survival rate in an animal model.Virus Genes. 2008 Feb;36(1):141-6. doi: 10.1007/s11262-007-0192-y. Epub 2008 Jan 3. Virus Genes. 2008. PMID: 18172750
-
Current Transport Systems and Clinical Applications for Small Interfering RNA (siRNA) Drugs.Mol Diagn Ther. 2018 Oct;22(5):551-569. doi: 10.1007/s40291-018-0338-8. Mol Diagn Ther. 2018. PMID: 29926308 Review.
-
Emerging nanotechnology approaches for HIV/AIDS treatment and prevention.Nanomedicine (Lond). 2010 Feb;5(2):269-85. doi: 10.2217/nnm.10.1. Nanomedicine (Lond). 2010. PMID: 20148638 Free PMC article. Review.
-
Lentivirus-mediated short hairpin RNA interference targeting TNF-alpha in macrophages inhibits particle-induced inflammation and osteolysis in vitro and in vivo.BMC Musculoskelet Disord. 2016 Oct 18;17(1):431. doi: 10.1186/s12891-016-1290-6. BMC Musculoskelet Disord. 2016. PMID: 27756280 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

