Skin human papillomavirus type 38 alters p53 functions by accumulation of deltaNp73
- PMID: 16397624
- PMCID: PMC1456898
- DOI: 10.1038/sj.embor.7400615
Skin human papillomavirus type 38 alters p53 functions by accumulation of deltaNp73
Abstract
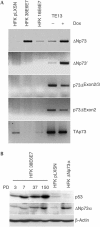
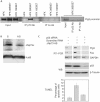
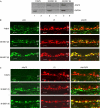
The E6 and E7 of the cutaneous human papillomavirus (HPV) type 38 immortalize primary human keratinocytes, an event normally associated with the inactivation of pathways controlled by the tumour suppressor p53. Here, we show for the first time that HPV38 alters p53 functions. Expression of HPV38 E6 and E7 in human keratinocytes or in the skin of transgenic mice induces stabilization of wild-type p53. This selectively activates the transcription of deltaNp73, an isoform of the p53-related protein p73, which in turn inhibits the capacity of p53 to induce the transcription of genes involved in growth suppression and apoptosis. DeltaNp73 downregulation by an antisense oligonucleotide leads to transcriptional re-activation of p53-regulated genes and apoptosis. Our findings illustrate a novel mechanism of the alteration of p53 function that is mediated by a cutaneous HPV type and support the role of HPV38 and deltaNp73 in human carcinogenesis.
Figures


References
-
- Allart S, Martin H, Detraves C, Terrasson J, Caput D, Davrinche C (2002) Human cytomegalovirus induces drug resistance and alteration of programmed cell death by accumulation of ΔN-p73α. J Biol Chem 277: 29063–29068 - PubMed
-
- Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805 - PubMed
-
- Caldeira S, Filotico R, Accardi R, Zehbe I, Franceschi S, Tommasino M (2004) p53 mutations are common in human papillomavirus type 38-positive non-melanoma skin cancers. Cancer Lett 209: 119–124 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

