Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study
- PMID: 16398939
- PMCID: PMC1361775
- DOI: 10.1186/1471-2407-6-5
Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study
Abstract
Background: Carboplatin/paclitaxel is the chemotherapy of choice for advanced ovarian cancer, both in first line and in platinum-sensitive recurrence. Although a significant proportion of patients have some neurotoxicity during treatment, the long-term outcome of chemotherapy-induced neuropathy has been scantly studied. We retrospectively assessed the prevalence of residual neuropathy in a cohort of patients in clinical remission after first-line carboplatin/paclitaxel for advanced ovarian cancer.
Methods: 120 patients have been included in this study (101 participating in a multicentre phase III trial evaluating the efficacy of consolidation treatment with topotecan, and 19 treated at the National Cancer Institute of Naples after the end of the trial). All patients received carboplatin (AUC 5) plus paclitaxel (175 mg/m2) every 3 weeks for 6 cycles, completing treatment between 1998 and 2003. Data were collected between May and September 2004. Residual sensory and motor neurotoxicity were coded according to the National Cancer Institute--Common Toxicity Criteria.
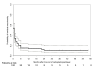
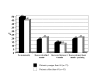
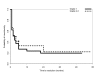
Results: 55 patients (46%) did not experience any grade of neurological toxicity during chemotherapy and of these none had signs of neuropathy during follow-up. The other 65 patients (54%) had chemotherapy-induced neurotoxicity during treatment and follow-up data are available for 60 of them. Fourteen out of 60 patients (23%) referred residual neuropathy at the most recent follow-up visit, after a median follow up of 18 months (range, 7-58 months): 12 patients had grade 1 and 2 patients grade 2 peripheral sensory neuropathy; 3 patients also had grade 1 motor neuropathy. The remaining 46/60 patients (77%) had no residual neuropathy at the moment of interview: recovery from neurotoxicity had occurred in the first 2 months after the end of chemotherapy in 22 (37%), between 2 and 6 months in 15 (25%), or after more than 6 months in 9 patients (15%). Considering all 120 treated patients, there was a 15% probability of persistent neurological toxicity 6 months after the end of chemotherapy.
Conclusion: A significant proportion of patients with advanced ovarian cancer treated with first-line carboplatin/paclitaxel suffer long-term residual neuropathy. This issue should be carefully taken into account before considering re-treatment with the same agents in sensitive recurrent disease.
Figures
Similar articles
-
Paclitaxel/carboplatin as first-line chemotherapy in advanced ovarian cancer: efficacy and adverse effects with special consideration of peripheral neurotoxicity.Anticancer Res. 2000 Sep-Oct;20(5C):4047-50. Anticancer Res. 2000. PMID: 11268499 Clinical Trial.
-
Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial.Lancet Oncol. 2014 Apr;15(4):396-405. doi: 10.1016/S1470-2045(14)70049-X. Epub 2014 Feb 28. Lancet Oncol. 2014. PMID: 24582486 Clinical Trial.
-
Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial.Lancet Oncol. 2015 Jan;16(1):87-97. doi: 10.1016/S1470-2045(14)71135-0. Epub 2014 Dec 4. Lancet Oncol. 2015. PMID: 25481791 Clinical Trial.
-
Medical therapy of advanced malignant epithelial tumours of the ovary.Forum (Genova). 2000 Oct-Dec;10(4):323-32. Forum (Genova). 2000. PMID: 11535983 Review.
-
Chemotherapy-induced neurotoxicity in the treatment of gynecological cancers: State of art and an innovative approach for prevention.World J Clin Oncol. 2021 Jun 24;12(6):458-467. doi: 10.5306/wjco.v12.i6.458. World J Clin Oncol. 2021. PMID: 34189069 Free PMC article. Review.
Cited by
-
Quantitative evaluation of chemotherapy-induced peripheral neuropathy by using intraepidermal electrical stimulation.Mol Clin Oncol. 2020 Aug;13(2):169-174. doi: 10.3892/mco.2020.2056. Epub 2020 Jun 3. Mol Clin Oncol. 2020. PMID: 32714541 Free PMC article.
-
The comparison of functional status and health-related parameters in ovarian cancer survivors with healthy controls.Support Care Cancer. 2024 Jan 22;32(2):119. doi: 10.1007/s00520-024-08311-x. Support Care Cancer. 2024. PMID: 38252310 Free PMC article.
-
Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy; cause and effect?Mol Pain. 2017 Jan-Dec;13:1744806917714693. doi: 10.1177/1744806917714693. Mol Pain. 2017. PMID: 28580836 Free PMC article.
-
Depletion of nerve growth factor in chemotherapy-induced peripheral neuropathy associated with hematologic malignancies.PLoS One. 2017 Aug 21;12(8):e0183491. doi: 10.1371/journal.pone.0183491. eCollection 2017. PLoS One. 2017. PMID: 28827818 Free PMC article.
-
Contemporary quality of life issues affecting gynecologic cancer survivors.Hematol Oncol Clin North Am. 2012 Feb;26(1):169-94. doi: 10.1016/j.hoc.2011.11.001. Epub 2011 Dec 16. Hematol Oncol Clin North Am. 2012. PMID: 22244668 Free PMC article. Review.
References
-
- du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J, Arbeitsgemeinschaft Gynakologische Onkologie Ovarian Cancer Study Group A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. - PubMed
-
- Neijt JP, Engelholm SA, Tuxen MK, Sorensen PG, Hansen M, Sessa C, de Swart CA, Hirsch FR, Lund B, van Houwelingen HC. Exploratory phase III study of cisplatin and paclitaxel versus carboplatin and paclitaxel in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R, Gynecologic Oncology Group Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. - DOI - PubMed
-
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Trope C, ICON and AGO Collaborators Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4 / AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/S0140-6736(03)13718-X. - DOI - PubMed
-
- National Cancer Institute – Cancer Therapy Evaluation Program. Common Toxicity Criteria. Version 2.0 April 30, 1999 http://ctep.info.nih.gov
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

