Neural modulation of cardiac arrhythmias and sudden cardiac death
- PMID: 16399065
- PMCID: PMC2566299
- DOI: 10.1016/j.hrthm.2005.09.021
Neural modulation of cardiac arrhythmias and sudden cardiac death
Figures




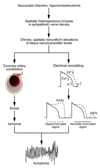
References
-
- Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004 Oct 15;95(8):754–763. - PubMed
-
- Rubart M, Zipes DP. Genes and cardiac repolarization: the challenge ahead. Circulation. 2005 Aug 30;112(9):1242–1244. - PubMed
-
- Priori S, Zipes DP. Sudden Cardiac Death. Elsevier Pub; 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

