Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol
- PMID: 16399829
- PMCID: PMC1403193
- DOI: 10.1529/biophysj.105.072801
Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol
Abstract
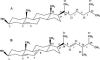
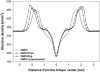
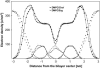
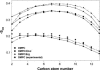
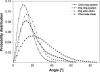
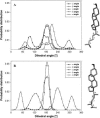
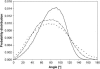
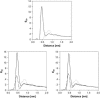
Sterol molecules are essential for maintaining the proper structure and function of eukaryotic cell membranes. The influence of cholesterol (the principal sterol of higher animals) on the lipid bilayer properties was extensively studied by both experimental and simulation methods. In contrast, the effect of ergosterol (the principal fungal sterol) on the membrane structure and dynamics is much less recognized. This work presents the results of comparative molecular dynamics simulation of the hydrated dimyristoylphosphatidylcholine bilayer containing approximately 25 mol % of cholesterol or ergosterol. A detailed analysis of the molecular properties (e.g., bilayer thickness, lipid order, diffusion, intermolecular interactions, etc.) of both sterol-induced liquid-ordered membrane phases is presented. Presence of sterols in the membrane significantly changes its property, especially fluidity and molecular packing. Moreover, in accordance with the experiments, our calculations show that, compared to cholesterol, ergosterol has higher ordering effect on the phospholipid acyl chains. This different influence on the properties of the lipid bilayer stems from differences in conformational freedom of sterol side chains. Additionally, obtained models of lipid membranes containing human and fungal sterols, constituting the result of our work, can be also utilized in other chemotherapeutic studies on interaction of selected ligands (e.g., antifungal compounds) with membranes.
Figures

References
-
- Simons, K., and E. Ikonen. 2000. Cell biology—how cells handle cholesterol. Science. 290:1721–1726. - PubMed
-
- McMullen, T. P. W., and R. N. McElhaney. 1996. Physical studies of cholesterol-phospholipid interactions. Curr. Opin. Colloid Interface Sci. 1:83–90.
-
- Ohvo-Rekila, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. - PubMed
-
- Trouard, T. P., A. A. Nevzorov, T. M. Alam, C. Job, J. Zajicek, and M. F. Brown. 1999. Influence of cholesterol on dynamics of dimyristoylphosphatidylcholine bilayers as studied by deuterium NMR relaxation. J. Chem. Phys. 110:8802–8818.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

