Single-channel water permeabilities of Escherichia coli aquaporins AqpZ and GlpF
- PMID: 16399837
- PMCID: PMC1403167
- DOI: 10.1529/biophysj.105.073965
Single-channel water permeabilities of Escherichia coli aquaporins AqpZ and GlpF
Abstract
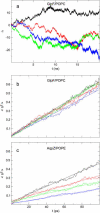
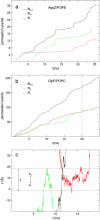
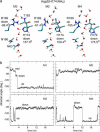
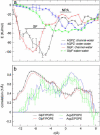
From equilibrium molecular dynamics simulations we have determined single-channel water permeabilities for Escherichia coli aquaporin Z (AqpZ) and aquaglyceroporin GlpF with the channels embedded in lipid bilayers. GlpF's osmotic water permeability constant pf exceeds by 2-3 times that of AqpZ and the diffusive permeability constant (pd) of GlpF is found to exceed that of AqpZ 2-9-fold. Achieving complete water selectivity in AqpZ consequently implies lower transport rates overall relative to the less selective, wider channel of GlpF. For AqpZ, the ratio pf/pd congruent with 12 is close to the average number of water molecules in the channel lumen, whereas for GlpF, pf/pd congruent with 4. This implies that single-file structure of the luminal water is more pronounced for AqpZ, the narrower channel of the two. Electrostatics profiles across the pore lumens reveal that AqpZ significantly reinforces water-channel interactions, and weaker water-water interactions in turn suppress water-water correlations relative to GlpF. Consequently, suppressed water-water correlations across the narrow selectivity filter become a key structural determinant for water permeation causing luminal water to permeate slower across AqpZ.
Figures



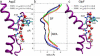
References
-
- Preston, G. M., P. Piazza-Carroll, W. B. Guggino, and P. Agre. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 water channel. Science. 256:385–387. - PubMed
-
- Agre, P. 1998. The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 273:14659–14662. - PubMed
-
- Borgnia, M., S. Nielsen, A. Engel, and P. Agre. 1999. a. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 68:425–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

