Severe bacteremia results in a loss of hepatic bacterial clearance
- PMID: 16399991
- PMCID: PMC2662948
- DOI: 10.1164/rccm.200509-1470OC
Severe bacteremia results in a loss of hepatic bacterial clearance
Abstract
Rationale: Although it has been postulated that liver injury results in impaired clearance of bacteria from the blood, no prior study has evaluated hepatic bacterial clearance during sepsis.
Objectives: We hypothesized that liver injury during the evolution of sepsis would result in impaired hepatic bacterial clearance.
Methods: Mild and severe bacteremia were generated in C57BL/6 mice by low- and high-dose intratracheal inoculation with Pseudomonas aeruginosa.
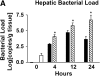
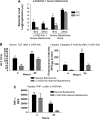
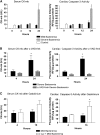
Measurements and main results: The mortality rates with mild and severe bacteremia were 20% and 60%, respectively. Hepatic bacterial clearance was preserved throughout the evolution of mild bacteremia but was lost late with severe bacteremia. The loss of hepatic bacterial clearance resulted in increased systemic bacteremia and mortality. Pretreatment with a caspase inhibitor resulted in preservation of hepatic bacterial clearance with severe bacteremia and eventual control of the bacteremia. When Kupffer cells were ablated before the onset of bacteremia, there was a loss of hepatic bacterial clearance. This converted an initially mild bacteremia into severe bacteremia with increased organ injury and mortality.
Conclusions: These observations suggest that hepatic bacterial clearance may be lost during the evolution of sepsis, resulting in a failure to control bacteremia. Thus, the capacity of the liver to clear bacteria is an important determinant of the outcome in sepsis.
Figures
















References
-
- Pittet D, Thievent B, Wenzel RP, Li N, Auckenthaler R, Suter PM. Bedside prediction of mortality from bacteremic sepsis: aA dynamic analysis of ICU patients. Am J Respir Crit Care Med 1996;153:684–693. - PubMed
-
- Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg 2000;7:40–48. - PubMed
-
- Graudal N, Milman N, Kirkegaard E, Korner B, Thomsen AC. Bacteremia in cirrhosis of the liver. Liver 1986;6:297–301. - PubMed
-
- Graudal N, Hubeck B, Bonde J, Thomsen AC. The prognostic significance of bacteremia in hepatic cirrhosis. Liver 1987;7:138–141. - PubMed
-
- Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Gimson A, Casewell M, Fagan E, Williams R. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology 1990;11:49–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

