Expression and function of C/EBP homology protein (GADD153) in podocytes
- PMID: 16400006
- PMCID: PMC1592667
- DOI: 10.2353/ajpath.2006.040774
Expression and function of C/EBP homology protein (GADD153) in podocytes
Abstract
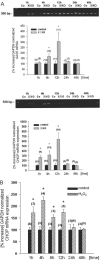
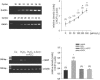
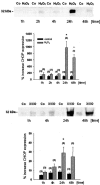
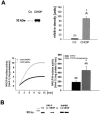
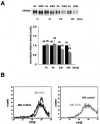
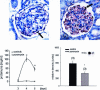
Podocytes are crucial for the permeability of the glomerular filtration barrier. In glomerular disease, however, reactive oxygen species (ROS) may be involved in podocyte injury and subsequent proteinuria. Here, we describe ROS-dependent gene induction in differentiated podocytes stimulated with H(2)O(2) or xanthine/xanthine-oxidase. Superoxide anions and H(2)O(2) increased mRNA and protein expression of GAS5 (growth arrest-specific protein 5) and CHOP (C/EBP homology protein). Cultured podocytes overexpressing CHOP showed increased generation of superoxide anions compared to controls. In addition, the expression of alpha(3)/beta(1) integrins, crucial for cell-matrix interaction of podocytes, was down-regulated, leading to increased cell-matrix adhesion and cell displacement. The altered cell-matrix adhesion was antagonized by the ROS scavenger 1,3-dimethyl-2-thiourea, and the increase in cell displacement could be mimicked by stimulating untransfected podocytes with puromycin, an inductor of ROS. We next performed immunohistochemical staining of human kidney tissue (normal, membranous nephropathy, focal segmental glomerulosclerosis, and minimal change nephropathy) as well as sections from rats with puromycin nephrosis, a model of minimal change nephropathy. CHOP was weakly expressed in podocytes of control kidneys but up-regulated in most proteinuric human kidneys and in rat puromycin nephrosis. Our data suggest that CHOP-via increased ROS generation-regulates cell-matrix adhesion of podocytes in glomerular disease.
Figures

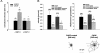

References
-
- Pavenstadt H. Roles of the podocyte in glomerular function. Am J Physiol. 2000;278:F173–F179. - PubMed
-
- Kerjaschki D, Neale TJ. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J Am Soc Nephrol. 1996;7:2518–2526. - PubMed
-
- Gwinner W, Grone HJ. Role of reactive oxygen species in glomerulonephritis. Nephrol Dial Transplant. 2000;15:1127–1132. - PubMed
-
- Yoshioka T, Ichikawa I, Fogo A. Reactive oxygen metabolites cause massive, reversible proteinuria and glomerular sieving defect without apparent ultrastructural abnormality. J Am Soc Nephrol. 1991;2:902–912. - PubMed
-
- Ricardo SD, Bertram JF, Ryan GB. Antioxidants protect podocyte foot processes in puromycin aminonucleoside-treated rats. J Am Soc Nephrol. 1994;4:1974–1986. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

