Chronic psychological stress in rats induces intestinal sensitization to luminal antigens
- PMID: 16400013
- PMCID: PMC1592661
- DOI: 10.2353/ajpath.2006.050575
Chronic psychological stress in rats induces intestinal sensitization to luminal antigens
Abstract
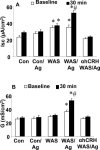
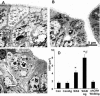
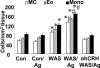
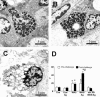
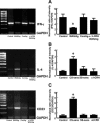
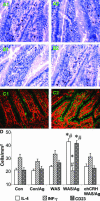
There is increasing evidence that stress plays a role in the pathophysiology of chronic intestinal disorders, but the mechanisms remain unclear. Previous studies in rats have revealed that stress decreases gut barrier function and allows excessive uptake of luminal material. Here, we investigated whether chronic psychological stress acts to induce sensitization of intestinal tissues to oral antigens. Rats were subjected to 1 hour per day of water avoidance stress or sham stress daily for 10 days, and horseradish peroxidase (HRP) was delivered by gavage on day 5. Studies to determine sensitization were conducted on day 20. All stressed rats developed HRP-specific IgE antibodies, antigen-induced intestinal secretion, and increased numbers of inflammatory cells in gut mucosa. Luminal HRP was absorbed more readily by enterocytes of stressed animals. In addition, stressed rats had increased expression of interleukin-4 and decreased expression of interferon-gamma in gut mucosa, a cytokine profile that is typical of allergic conditions. Treatment of stressed rats with an antagonist to corticotropin-releasing hormone (previously shown to inhibit stress-enhanced gut permeability) eliminated the manifestations of intestinal hypersensitivity. Our results indicate that the presence of oral antigen during chronic psychological stress alters the immune response (to sensitization rather than oral tolerance) and causes subsequent antigen-induced gut pathophysiology.
Figures
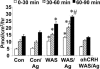
Comment in
-
How stress induces intestinal hypersensitivity.Am J Pathol. 2006 Jan;168(1):3-5. doi: 10.2353/ajpath.2006.050958. Am J Pathol. 2006. PMID: 16400003 Free PMC article. Review. No abstract available.
References
-
- Collins SM. Stress and the gastrointestinal tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol. 2001;280:G315–G318. - PubMed
-
- Monnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Monnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201–211. - PubMed
-
- Hart A, Kamm MA. Mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment Pharmacol Ther. 2002;16:2017–2028. - PubMed
-
- Kelsay K. Psychological aspects of food allergy. Curr Allergy Asthma Rep. 2003;3:41–46. - PubMed
-
- Guarner F, Casellas F, Borruel N, Antolin M, Videla S, Vilaseca J, Malagelada JR. Role of microecology in chronic inflammatory bowel diseases. Eur J Clin Nutr. 2002;56:S34–S38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

