Caveolin-1 deficiency (-/-) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness
- PMID: 16400031
- PMCID: PMC1592656
- DOI: 10.2353/ajpath.2006.050429
Caveolin-1 deficiency (-/-) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness
Abstract
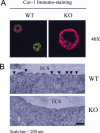
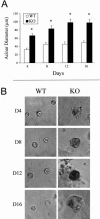
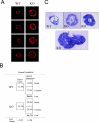
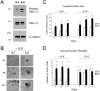
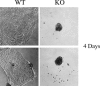
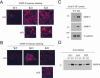
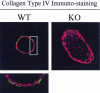
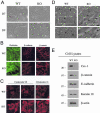
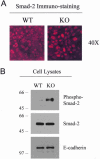
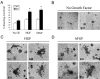
During breast cancer development, the luminal space of the mammary acinar unit fills with proliferating epithelial cells that exhibit growth factor-independence, cell attachment defects, and a more invasive fibroblastic phenotype. Here, we used primary cultures of mammary epithelial cells derived from genetically engineered mice to identify caveolin-1 (Cav-1) as a critical factor for maintaining the normal architecture of the mammary acinar unit. Isolated cultures of normal mammary epithelial cells retained the capacity to generate mammary acini within extracellular matrix. However, those from Cav-1 (-/-) mice exhibited defects in three-dimensional acinar architecture, including disrupted lumen formation and epidermal growth factor-independent growth due to hyperactivation of the p42/44 mitogen-activated protein kinase cascade. In addition, Cav-1-null mammary epithelial cells deprived of exogenous extracellular matrix underwent a spontaneous epithelial-mesenchymal transition, with reorganization of the actin cytoskeleton, and E-cadherin redistribution. Mechanistically, these phenotypic changes appear to be caused by increases in matrix metalloproteinase-2/9 secretion and transforming growth factor-beta/Smad-2 hyperactivation. Finally, loss of Cav-1 potentiated the ability of growth factors (hepatocyte growth factor and basic fibroblast growth factor) to induce mammary acini branching, indicative of a more invasive fibroblastic phenotype. Thus, a Cav-1 deficiency profoundly affects mammary epithelia by modulating the activation state of important signaling cascades. Primary cultures of Cav-1-deficient mammary epithelia will provide a valuable new model to study the spatial/temporal progression of mammary cell transformation.
Figures
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. - PubMed
-
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. - PubMed
-
- Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

