Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2
- PMID: 16401081
- PMCID: PMC2851202
- DOI: 10.1021/bi051235w
Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2
Abstract
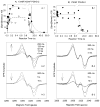
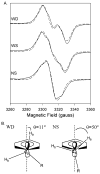
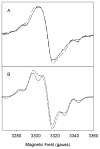
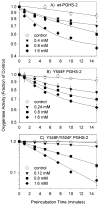
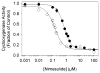
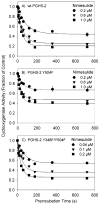
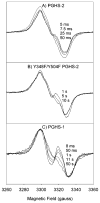
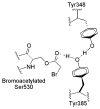
Both prostaglandin H synthase (PGHS) isoforms utilize a radical at Tyr385 to abstract a hydrogen atom from arachidonic acid, initializing prostaglandin synthesis. A Tyr348-Tyr385 hydrogen bond appears to be conserved in both isoforms; this hydrogen bonding has the potential to modulate the positioning and reactivity of the Tyr385 side chain. The EPR signal from the Tyr385 radical undergoes a time-dependent transition from a wide doublet to a wide singlet species in both isoforms. In PGHS-2, this transition results from radical migration from Tyr385 to Tyr504. Localization of the radical to Tyr385 in the recombinant human PGHS-2 Y504F mutant was exploited in examining the effects of blocking Tyr385 hydrogen bonding by introduction of a further Y348F mutation. Cyclooxygenase and peroxidase activities were found to be maintained in the Y348F/Y504F mutant, but the Tyr385 radical was formed more slowly and had greater rotational freedom, as evidenced by observation of a transition from an initial wide doublet species to a narrow singlet species, a transition not seen in the parent Y504F mutant. The effect of disrupting Tyr385 hydrogen bonding on the cyclooxygenase active site structure was probed by examination of cyclooxygenase inhibitor kinetics. Aspirin treatment eliminated all oxygenase activity in the Y348F/Y504F double mutant, with no indication of the lipoxygenase activity observed in aspirin-treated wild-type PGHS-2. Introduction of the Y348F mutation also strengthened the time-dependent inhibitory action of nimesulide. These results suggest that removal of Tyr348-Tyr385 hydrogen bonding in PGHS-2 allows greater conformational flexibility in the cyclooxygenase active site, resulting in altered interactions with inhibitors and altered Tyr385 radical behavior.
Figures

References
-
- Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. - PubMed
-
- Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–140. - PubMed
-
- Lambeir AM, Markey CM, Dunford HB, Marnett LJ. Spectral properties of the higher oxidation states of prostaglandin H synthase. J Biol Chem. 1985;260:14894–14896. - PubMed
-
- Tang MS, Copeland RA, Penning TM. Detection of an Fe2+-protoporphyrin-IX intermediate during aspirin-treated prostaglandin H2 synthase II catalysis of arachidonic acid to 15-HETE. Biochemistry. 1997;36:7527–7534. - PubMed
-
- Lu G, Tsai AL, Van Wart HE, Kulmacz RJ. Comparison of the peroxidase reaction kinetics of prostaglandin H synthase-1 and -2. J Biol Chem. 1999;274:16162–16167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

