Radical intermediates in the catalytic oxidation of hydrocarbons by bacterial and human cytochrome P450 enzymes
- PMID: 16401082
- PMCID: PMC2566308
- DOI: 10.1021/bi051840z
Radical intermediates in the catalytic oxidation of hydrocarbons by bacterial and human cytochrome P450 enzymes
Abstract
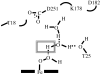
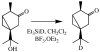
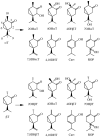
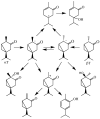
Cytochromes P450cam and P450BM3 oxidize alpha- and beta-thujone into multiple products, including 7-hydroxy-alpha-(or beta-)thujone, 7,8-dehydro-alpha-(or beta-)thujone, 4-hydroxy-alpha-(or beta-)thujone, 2-hydroxy-alpha-(or beta-)thujone, 5-hydroxy-5-isopropyl-2-methyl-2-cyclohexen-1-one, 4,10-dehydrothujone, and carvacrol. Quantitative analysis of the 4-hydroxylated isomers and the ring-opened product indicates that the hydroxylation proceeds via a radical mechanism with a radical recombination rate ranging from 0.7 +/- 0.3 x 10(10) s(-1) to 12.5 +/- 3 x 10(10) s(-1) for the trapping of the carbon radical by the iron-bound hydroxyl radical equivalent. 7-[2H]-alpha-Thujone has been synthesized and used to amplify C-4 hydroxylation in situations where uninformative C-7 hydroxylation is the dominant reaction. The involvement of a carbon radical intermediate is confirmed by the observation of inversion of stereochemistry of the methyl-substituted C-4 carbon during the hydroxylation. With an L244A mutation that slightly increases the P450(cam) active-site volume, this inversion is observed in up to 40% of the C-4 hydroxylated products. The oxidation of alpha-thujone by human CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 occurs with up to 80% C-4 methyl inversion, in agreement with a dominant radical hydroxylation mechanism. Three minor desaturation products are produced, with at least one of them via a cationic pathway. The cation involved is proposed to form by electron abstraction from a radical intermediate. The absence of a solvent deuterium isotope effect on product distribution in the P450cam reaction precludes a significant role for the P450 ferric hydroperoxide intermediate in substrate hydroxylation. The results indicate that carbon hydroxylation is catalyzed exclusively by a P450 ferryl species via radical intermediates whose detailed properties are substrate- and enzyme-dependent.
Figures
References
-
- Kagawa N, Waterman MR. Regulation of steroidogenic and related P450s. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 2nd Ed. Plenum Press; New York: 1995. pp. 419–442.
-
- Capdevila JH. Cytochrome P450 and the metabolism and bioactivation of arachidonic acid and eicosanoids. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd Ed. Plenum Klewer; New York: 2005. pp. 531–551.
-
- Groves JT, McGlusky GA, White RE, Coon MJ. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P450. Evidence for a carbon radical intermediate. Biochem. Biophys. Res. Commun. 1978;81:154–160. - PubMed
-
- Ortiz de Montellano PR, De Voss JJ. Substrate oxidation by cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd Ed. Plenum Klewer; New York: 2005. pp. 183–245.
-
- Makris TM, Denisov I, Schlichting I, Sligar SG. Activation of molecular oxygen by cytochrome P450. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd Ed. Plenum Klewer; New York: 2005. pp. 149–182.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

