Facilitating RNA structure prediction with microarrays
- PMID: 16401087
- PMCID: PMC4070881
- DOI: 10.1021/bi051409+
Facilitating RNA structure prediction with microarrays
Abstract
Determining RNA secondary structure is important for understanding structure-function relationships and identifying potential drug targets. This paper reports the use of microarrays with heptamer 2'-O-methyl oligoribonucleotides to probe the secondary structure of an RNA and thereby improve the prediction of that secondary structure. When experimental constraints from hybridization results are added to a free-energy minimization algorithm, the prediction of the secondary structure of Escherichia coli 5S rRNA improves from 27 to 92% of the known canonical base pairs. Optimization of buffer conditions for hybridization and application of 2'-O-methyl-2-thiouridine to enhance binding and improve discrimination between AU and GU pairs are also described. The results suggest that probing RNA with oligonucleotide microarrays can facilitate determination of secondary structure.
Figures
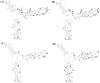

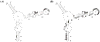
 - middle site of strong binding;
- middle site of strong binding;
 - middle site of medium binding.
- middle site of medium binding.

 - middle site of strong binding;
- middle site of strong binding;
 - middle site of medium binding.
- middle site of medium binding.Similar articles
-
Interpreting oligonucleotide microarray data to determine RNA secondary structure: application to the 3' end of Bombyx mori R2 RNA.Biochemistry. 2006 Aug 15;45(32):9819-32. doi: 10.1021/bi052618x. Biochemistry. 2006. PMID: 16893182
-
The calculation of plant 5S rRNAs secondary structure.Acta Biochim Pol. 1989;36(3-4):215-23. Acta Biochim Pol. 1989. PMID: 2485998
-
The 3D arrangement of the 23 S and 5 S rRNA in the Escherichia coli 50 S ribosomal subunit based on a cryo-electron microscopic reconstruction at 7.5 A resolution.J Mol Biol. 2000 Apr 21;298(1):35-59. doi: 10.1006/jmbi.2000.3635. J Mol Biol. 2000. PMID: 10756104
-
[Structure and function of 5S rRNA in ribosomes].Mol Biol (Mosk). 1995 Nov-Dec;29(6):1218-27. Mol Biol (Mosk). 1995. PMID: 8592496 Review. Russian. No abstract available.
-
Prediction of RNA secondary structure by free energy minimization.Curr Opin Struct Biol. 2006 Jun;16(3):270-8. doi: 10.1016/j.sbi.2006.05.010. Epub 2006 May 19. Curr Opin Struct Biol. 2006. PMID: 16713706 Review.
Cited by
-
Enzymatic synthesis of structure-free DNA with pseudo-complementary properties.Nucleic Acids Res. 2008 Jun;36(10):3409-19. doi: 10.1093/nar/gkn209. Epub 2008 Apr 29. Nucleic Acids Res. 2008. PMID: 18448471 Free PMC article.
-
NMR-assisted prediction of RNA secondary structure: identification of a probable pseudoknot in the coding region of an R2 retrotransposon.J Am Chem Soc. 2008 Aug 6;130(31):10233-9. doi: 10.1021/ja8026696. Epub 2008 Jul 10. J Am Chem Soc. 2008. PMID: 18613678 Free PMC article.
-
Secondary structures for 5' regions of R2 retrotransposon RNAs reveal a novel conserved pseudoknot and regions that evolve under different constraints.J Mol Biol. 2009 Jul 17;390(3):428-42. doi: 10.1016/j.jmb.2009.04.048. Epub 2009 May 3. J Mol Biol. 2009. PMID: 19397915 Free PMC article.
-
Measuring intramolecular connectivity in long RNA molecules using two-dimensional DNA patch-probe arrays.Nucleic Acids Res. 2025 Jun 6;53(11):gkaf469. doi: 10.1093/nar/gkaf469. Nucleic Acids Res. 2025. PMID: 40479708 Free PMC article.
-
Structural determinants for alternative splicing regulation of the MAPT pre-mRNA.RNA Biol. 2015;12(3):330-42. doi: 10.1080/15476286.2015.1017214. RNA Biol. 2015. PMID: 25826665 Free PMC article.
References
-
- Fox GE, Woese CR. 5S-RNA secondary structure. Nature. 1975;256:505–507. - PubMed
-
- Pace NR, Thomas BC, Woese CR. Probing RNA structure, function, and history by comparative analysis. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. Cold Spring Harbor Press; New York: 1999. pp. 113–141.
-
- Goertzen LR, Cannone JJ, Gutell RR, Jansen RK. ITS secondary structure derived from comparative analysis: implications for sequence alignment and phylogeny of the Asteraceae. Molecular Phylogenetics and Evolution. 2003;29:216–234. - PubMed
-
- Cannone JJ, Subramanian S, Schnare MN, Collett JR, D’Souza LM, Du YS, Feng B, Lin N, Madabusi LV, Muller KM, Pande N, Shang ZD, Yu N, Gutell RR. The Comparative RNA Web (CRW) Site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. Bmc Bioinformatics. 2002:3. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

