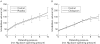A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome
- PMID: 16401691
- PMCID: PMC1856276
- DOI: 10.1136/gut.2005.077503
A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome
Abstract
Introduction: Selective serotonin reuptake inhibitors (SSRIs) are frequently used in the treatment of irritable bowel syndrome (IBS) although evidence of their efficacy is scarce.
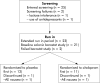
Aim: Twenty three non-depressed IBS patients were recruited from a tertiary care centre and included in a crossover trial comparing six weeks of treatment with the SSRI citalopram (20 mg for three weeks, 40 mg for three weeks) with placebo. IBS symptom severity was the primary outcome measure, and depression and anxiety scores were also measured. The effect of acute administration of citalopram on colonic sensitivity and on colonic response to feeding was investigated as a putative predictor of symptomatic response to the drug.
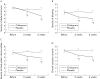
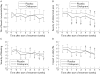
Results: After three and six weeks of treatment, citalopram significantly improved abdominal pain, bloating, impact of symptoms on daily life, and overall well being compared with placebo. There was only a modest effect on stool pattern. Changes in depression or anxiety scores were not related to symptom improvement. The effect of acute administration of citalopram during a colonic barostat study did not predict clinical outcome. Analysis of the first treatment period as a double blind parallel arm study confirmed the benefit of citalopram over placebo.
Conclusions: The SSRI citalopram significantly improves IBS symptoms, including abdominal pain, compared with placebo. The therapeutic effect is independent of effects on anxiety, depression, and colonic sensorimotor function.
Conflict of interest statement
Conflict of interest: None declared.
Comment in
-
How do SSRIs help patients with irritable bowel syndrome?Gut. 2006 Aug;55(8):1065-7. doi: 10.1136/gut.2005.086348. Gut. 2006. PMID: 16849340 Free PMC article. Review.
-
Further evidence supporting a psychological component to irritable bowel syndrome.Gut. 2007 Mar;56(3):437-8; author reply 438. Gut. 2007. PMID: 17339251 Free PMC article. No abstract available.
-
The role of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome.Gut. 2007 May;56(5):733; author reply 733. Gut. 2007. PMID: 17440190 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical