An intramolecular t-SNARE complex functions in vivo without the syntaxin NH2-terminal regulatory domain
- PMID: 16401725
- PMCID: PMC2063558
- DOI: 10.1083/jcb.200507138
An intramolecular t-SNARE complex functions in vivo without the syntaxin NH2-terminal regulatory domain
Abstract
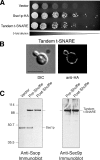
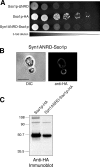
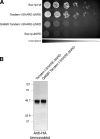
Membrane fusion in the secretory pathway is mediated by SNAREs (located on the vesicle membrane [v-SNARE] and the target membrane [t-SNARE]). In all cases examined, t-SNARE function is provided as a three-helix bundle complex containing three approximately 70-amino acid SNARE motifs. One SNARE motif is provided by a syntaxin family member (the t-SNARE heavy chain), and the other two helices are contributed by additional t-SNARE light chains. The syntaxin family is the most conformationally dynamic group of SNAREs and appears to be the major focus of SNARE regulation. An NH2-terminal region of plasma membrane syntaxins has been assigned as a negative regulatory element in vitro. This region is absolutely required for syntaxin function in vivo. We now show that the required function of the NH2-terminal regulatory domain (NRD) of the yeast plasma membrane syntaxin, Sso1p, can be circumvented when t-SNARE complex formation is made intramolecular. Our results suggest that the NRD is required for efficient t-SNARE complex formation and does not recruit necessary scaffolding factors.
Figures





Similar articles
-
The polybasic juxtamembrane region of Sso1p is required for SNARE function in vivo.Eukaryot Cell. 2005 Dec;4(12):2017-28. doi: 10.1128/EC.4.12.2017-2028.2005. Eukaryot Cell. 2005. PMID: 16339720 Free PMC article.
-
Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking.Traffic. 2006 Oct;7(10):1408-19. doi: 10.1111/j.1600-0854.2006.00474.x. Epub 2006 Aug 10. Traffic. 2006. PMID: 16899085
-
Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion.EMBO J. 2007 Dec 12;26(24):4935-45. doi: 10.1038/sj.emboj.7601915. Epub 2007 Nov 15. EMBO J. 2007. PMID: 18007597 Free PMC article.
-
Role of SNAREs in membrane fusion.Adv Exp Med Biol. 2011;713:13-32. doi: 10.1007/978-94-007-0763-4_3. Adv Exp Med Biol. 2011. PMID: 21432012 Review.
-
Decoding the interactions of SM proteins with SNAREs.ScientificWorldJournal. 2005 Jun 8;5:471-7. doi: 10.1100/tsw.2005.53. ScientificWorldJournal. 2005. PMID: 15962202 Free PMC article. Review.
Cited by
-
The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening.Nat Struct Mol Biol. 2010 Jun;17(6):710-7. doi: 10.1038/nsmb.1809. Epub 2010 May 9. Nat Struct Mol Biol. 2010. PMID: 20453860
-
Identifying eIF4E-binding protein translationally-controlled transcripts reveals links to mRNAs bound by specific PUF proteins.Nucleic Acids Res. 2010 Dec;38(22):8039-50. doi: 10.1093/nar/gkq686. Epub 2010 Aug 12. Nucleic Acids Res. 2010. PMID: 20705650 Free PMC article.
-
Sec3 promotes the initial binary t-SNARE complex assembly and membrane fusion.Nat Commun. 2017 Jan 23;8:14236. doi: 10.1038/ncomms14236. Nat Commun. 2017. PMID: 28112172 Free PMC article.
-
The polybasic juxtamembrane region of Sso1p is required for SNARE function in vivo.Eukaryot Cell. 2005 Dec;4(12):2017-28. doi: 10.1128/EC.4.12.2017-2028.2005. Eukaryot Cell. 2005. PMID: 16339720 Free PMC article.
References
-
- Bennett, W.F., N.F. Paoni, B.A. Keyt, D. Botstein, A.J. Jones, L. Presta, F.M. Wurm, and M.J. Zoller. 1991. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J. Biol. Chem. 266:5191–5201. - PubMed
-
- Burke, D., D. Dawson, and T. Stearns. 2000. Methods in Yeast Genetics: a Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. xvii, 205 pp.
-
- Calakos, N., M.K. Bennett, K.E. Peterson, and R.H. Scheller. 1994. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 263:1146–1149. - PubMed
-
- Chen, Y.A., and R.H. Scheller. 2001. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2:98–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

