Inhibition of autophosphorylation of epidermal growth factor receptor by small peptides in vitro
- PMID: 16402038
- PMCID: PMC1616988
- DOI: 10.1038/sj.bjp.0706634
Inhibition of autophosphorylation of epidermal growth factor receptor by small peptides in vitro
Abstract
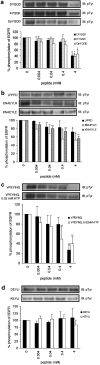
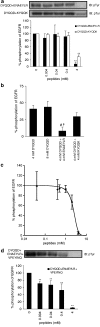
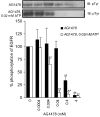
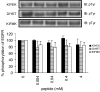
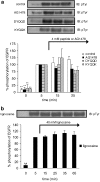
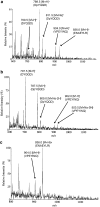
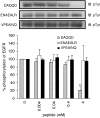
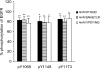
1. Inhibition of uncontrolled epidermal growth factor receptor (EGFR) is one of the approaches for the treatment of breast and lung cancers. We designed oligopeptides consisting of amino-acid sequences of the major (Y1068, Y1148, and Y1173) and minor (Y992) autophosphorylation sites of EGFR. These peptides may be exogenous substrates or pseudosubstrates that interfere with the autophosphorylation of EGFR. The effects of the peptides on autophosphorylation of EGFR were studied. 2. Purified EGFR was phosphorylated in vitro with EGF in the presence of various synthetic peptides. The phosphorylation level of EGFR was then evaluated after SDS-PAGE separation, followed by Western blot analysis with antiphosphotyrosine antibody. 3. Ac-VPEYINQ-NH2 (Y1068) and Ac-DYQQD-NH2 (Y1148) showed the most potent inhibitory effects, followed by Ac-ENAEYLR-NH2 (Y1173). These peptides at 4 mM suppressed phosphorylation to 30-50%. 4. Combination of the three kinds of peptides much more strongly inhibited autophosphorylation. The 50% inhibitory concentration (IC50) value was 0.5 mM as a mixture and was comparable to that of AG1478 (IC50, 0.3 mM) at 0.2 mM ATP. 5. Neither Ac-DIYET-NH2 or Ac-KIYEK-NH2, designed previously based on the amino-acid sequence of an autophosphorylation site of insulin receptor, nor their related (Ac-KIFMK-NH2) or unrelated (Ac-LPFFD-NH2) peptides showed an inhibitory effect. These results suggest that the small peptides that originated from the autophosphorylation sites of EGFR interact solely with EGFR. 6. The peptides containing the sequences surrounding Y1068, Y1148, and Y1173 may be a promising seed for the development of therapeutic agents for breast and lung cancers.
Figures
Similar articles
-
Inhibition of autophosphorylation of epidermal growth factor receptor by a small peptide not employing an ATP-competitive mechanism.Biopolymers. 2008 Jan;89(1):40-51. doi: 10.1002/bip.20843. Biopolymers. 2008. PMID: 17849478
-
Suppression of insulin signalling by a synthetic peptide KIFMK suggests the cytoplasmic linker between DIII-S6 and DIV-S1 as a local anaesthetic binding site on the sodium channel.Br J Pharmacol. 2004 May;142(1):222-8. doi: 10.1038/sj.bjp.0705575. Epub 2004 Mar 22. Br J Pharmacol. 2004. PMID: 15037518 Free PMC article.
-
Oligopeptides derived from autophosphorylation sites of EGF receptor suppress EGF-stimulated responses in human lung carcinoma A549 cells.Eur J Pharmacol. 2013 Jan 5;698(1-3):87-94. doi: 10.1016/j.ejphar.2012.10.007. Epub 2012 Oct 17. Eur J Pharmacol. 2013. PMID: 23085023
-
Synthetic pentapeptides inhibiting autophosphorylation of insulin receptor in a non-ATP-competitive mechanism.J Pept Sci. 2009 May;15(5):327-36. doi: 10.1002/psc.1114. J Pept Sci. 2009. PMID: 19206072
-
[Structure and function of the insulin receptor].Tanpakushitsu Kakusan Koso. 1984 Jul;29(7):533-50. Tanpakushitsu Kakusan Koso. 1984. PMID: 6093194 Review. Japanese. No abstract available.
Cited by
-
Exploring the Therapeutic Implications of Co-Targeting the EGFR and Spindle Assembly Checkpoint Pathways in Oral Cancer.Pharmaceutics. 2024 Sep 11;16(9):1196. doi: 10.3390/pharmaceutics16091196. Pharmaceutics. 2024. PMID: 39339232 Free PMC article. Review.
-
Inhibition of Phosphatidylcholine-Specific Phospholipase C Interferes with Proliferation and Survival of Tumor Initiating Cells in Squamous Cell Carcinoma.PLoS One. 2015 Sep 24;10(9):e0136120. doi: 10.1371/journal.pone.0136120. eCollection 2015. PLoS One. 2015. PMID: 26402860 Free PMC article.
-
EGFR-c-Src-Mediated HDAC3 Phosphorylation Exacerbates Invasion of Breast Cancer Cells.Cells. 2019 Aug 19;8(8):930. doi: 10.3390/cells8080930. Cells. 2019. PMID: 31430896 Free PMC article.
-
Src and epidermal growth factor receptor mediate the pro-invasive activity of Bcl-w.Tumour Biol. 2016 Jan;37(1):1245-52. doi: 10.1007/s13277-015-3917-x. Epub 2015 Aug 20. Tumour Biol. 2016. PMID: 26286833
-
The impact of molecular tumor profiling on the design strategies for targeting myeloid leukemia and EGFR/CD44-positive solid tumors.Beilstein J Nanotechnol. 2021 Apr 29;12:375-401. doi: 10.3762/bjnano.12.31. eCollection 2021. Beilstein J Nanotechnol. 2021. PMID: 33981532 Free PMC article. Review.
References
-
- BASELGA J. The science of EGFR inhibition: A roadmap to improved outcomes. Signal. 2004;5:4–8.
-
- BLAKESLEY V.A., SCRIMGEOUR A., ESPOSITO D., ROITH D.L. Signaling via the insulin-like growth factor-I receptor: Does it differ from insulin receptor signaling. Cytokine Growth Factor Rev. 1996;7:153–159. - PubMed
-
- CHANTRY A. The kinase domain and membrane localization determine intracellular interactions between epidermal growth factor receptors. J. Biol. Chem. 1995;270:3068–3073. - PubMed
-
- DOWNWARD J., PARKER P., WATERFIELD M.D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984;311:483–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

