Sphingolipids differentially regulate mitogen-activated protein kinases and intracellular Ca2+ in vascular smooth muscle: effects on CREB activation
- PMID: 16402047
- PMCID: PMC1616991
- DOI: 10.1038/sj.bjp.0706600
Sphingolipids differentially regulate mitogen-activated protein kinases and intracellular Ca2+ in vascular smooth muscle: effects on CREB activation
Abstract
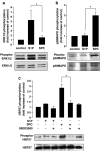
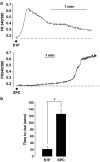
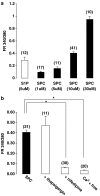
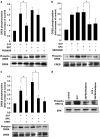
1. Related sphingolipids, sphingosine 1-phosphate (S1P) and sphingosylphosphorylcholine (SPC), have important effects on vascular smooth muscle. The aim of this study was to investigate the intracellular pathways regulated by S1P and SPC in rat cerebral artery. 2. In cerebral arteries, S1P increased extracellular signal-regulated kinase (ERK)1/2 phosphorylation (5.2+/-1.4-fold increase) but did not activate p38 mitogen-activated protein kinase (p38MAPK) as assessed by immunoblotting. In contrast, SPC increased p38MAPK phosphorylation (3.0+/-0.3-fold increase) but did not stimulate ERK1/2. This differential activation was confirmed by measuring activation of heat shock protein (HSP) 27, a known downstream target of p38MAPK. Only SPC, but not S1P, activated HSP27. 3. In enzymatically dispersed cerebral artery myocytes, SPC increased [Ca2+]i in a concentration-dependent manner (peak response at 10 microM: 0.4+/-0.02 ratio units) as determined using the Ca2+ indicator, Fura 2. In contrast to S1P, the SPC-induced [Ca2+]i increase did not involve intracellular release but was due to Ca2+ influx via L-type Ca2+ channels. 4. Despite differences in signalling, both S1P and SPC phosphorylated the transcription factor cAMP response element-binding protein (CREB). S1P-induced CREB activation was dependent on ERK1/2 and Ca2+-calmodulin-dependent protein kinase (CaMK) activation. CREB activation by SPC required both p38MAPK and CaMK activation, but not ERK1/2. 5. In conclusion, S1P and SPC activate distinct MAP kinase isoforms and increase [Ca2+]i via different mechanisms in rat cerebral artery. This does not affect the ability of S1P or SPC to activate CREB, although this occurs via different pathways.
Figures

Comment in
-
Sphingosine-1-phosphate and sphingosylphosphorylcholine: two of a kind?Br J Pharmacol. 2006 Feb;147(4):347-8. doi: 10.1038/sj.bjp.0706602. Br J Pharmacol. 2006. PMID: 16402045 Free PMC article.
Similar articles
-
Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels.Biochem Pharmacol. 2003 Nov 1;66(9):1861-70. doi: 10.1016/s0006-2952(03)00546-x. Biochem Pharmacol. 2003. PMID: 14563496
-
Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery.Cardiovasc Res. 2009 May 1;82(2):324-32. doi: 10.1093/cvr/cvp054. Epub 2009 Feb 13. Cardiovasc Res. 2009. PMID: 19218288
-
Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine.Naunyn Schmiedebergs Arch Pharmacol. 2006 Apr;373(1):18-29. doi: 10.1007/s00210-006-0046-5. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16570136 Review.
-
Ca2+ signaling induced by sphingosylphosphorylcholine and sphingosine 1-phosphate via distinct mechanisms in rat glomerular mesangial cells.Kidney Int. 1998 Nov;54(5):1470-83. doi: 10.1046/j.1523-1755.1998.00162.x. Kidney Int. 1998. PMID: 9844123
-
Roles of Cyclic AMP Response Element Binding Activation in the ERK1/2 and p38 MAPK Signalling Pathway in Central Nervous System, Cardiovascular System, Osteoclast Differentiation and Mucin and Cytokine Production.Int J Mol Sci. 2019 Mar 17;20(6):1346. doi: 10.3390/ijms20061346. Int J Mol Sci. 2019. PMID: 30884895 Free PMC article. Review.
Cited by
-
The dynamics and role of sphingolipids in eukaryotic organisms upon thermal adaptation.Prog Lipid Res. 2020 Nov;80:101063. doi: 10.1016/j.plipres.2020.101063. Epub 2020 Sep 2. Prog Lipid Res. 2020. PMID: 32888959 Free PMC article. Review.
-
Indomethacin differentiates the renal effects of sphingosine-1-phosphate and sphingosylphosphorylcholine.Naunyn Schmiedebergs Arch Pharmacol. 2006 Apr;373(1):37-44. doi: 10.1007/s00210-006-0037-6. Epub 2006 Mar 7. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16521006
-
Apoptotic cell-derived factors induce arginase II expression in murine macrophages by activating ERK5/CREB.Cell Mol Life Sci. 2011 May;68(10):1815-27. doi: 10.1007/s00018-010-0537-x. Epub 2010 Oct 15. Cell Mol Life Sci. 2011. PMID: 20949368 Free PMC article.
-
Development of a sphingosylphosphorylcholine detection system using RNA aptamers.Molecules. 2010 Aug 20;15(8):5742-55. doi: 10.3390/molecules15085742. Molecules. 2010. PMID: 20729797 Free PMC article.
-
Ginsenoside Rg1 attenuates hypoxia and hypercapnia-induced vasoconstriction in isolated rat pulmonary arterial rings by reducing the expression of p38.J Thorac Dis. 2016 Jul;8(7):1513-23. doi: 10.21037/jtd.2016.05.71. J Thorac Dis. 2016. PMID: 27499938 Free PMC article.
References
-
- AN S., BLEU T., ZHENG Y. Transduction of intracellular calcium signals through G-protein medicated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol. Pharmacol. 1999;55:787–794. - PubMed
-
- BEKTAS M., BARAK L.S., JOLLY P.S., LIU H., LYNCH K.R., LACANA E., SUHR K.B., MILSTEIN S., SPIEGEL S. The G protein-coupled receptor GPR4 suppresses ERK activation in a ligand-independent manner. Biochemistry. 2003;42:12181–12189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

