Heterotrimeric G-proteins: a short history
- PMID: 16402120
- PMCID: PMC1760735
- DOI: 10.1038/sj.bjp.0706405
Heterotrimeric G-proteins: a short history
Abstract
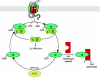
Some 865 genes in man encode G-protein-coupled receptors (GPCRs). The heterotrimeric guanine nucleotide-binding proteins (G-proteins) function to transduce signals from this vast panoply of receptors to effector systems including ion channels and enzymes that alter the rate of production, release or degradation of intracellular second messengers. However, it was not until the 1970s that the existence of such transducing proteins was even seriously suggested. Combinations of bacterial toxins that mediate their effects via covalent modification of the alpha-subunit of certain G-proteins and mutant cell lines that fail to generate cyclic AMP in response to agonists because they either fail to express or express a malfunctional G-protein allowed their identification and purification. Subsequent to initial cloning efforts, cloning by homology has defined the human G-proteins to derive from 35 genes, 16 encoding alpha-subunits, five beta and 14 gamma. All function as guanine nucleotide exchange on-off switches and are mechanistically similar to other proteins that are enzymic GTPases. Although not readily accepted initially, it is now well established that beta/gamma complexes mediate as least as many functions as the alpha-subunits. The generation of chimeras between different alpha-subunits defined the role of different sections of the primary/secondary sequence and crystal structures and cocrystals with interacting proteins have given detailed understanding of their molecular structure and basis of function. Finally, further modifications of such chimeras have generated a range of G-protein alpha-subunits with greater promiscuity to interact across GPCR classes and initiated the use of such modified G-proteins in drug discovery programmes.
Figures

References
-
- BAHIA D.S., WISE A., FANELLI F., LEE M., REES S., MILLIGAN G. Hydrophobicity of residue351 of the G-protein Gi1 alpha determines the extent of activation by the alpha2A-adrenoceptor. Biochemistry. 1998;37:11555–11562. - PubMed
-
- BARNES W.G., REITER E., VIOLIN J.D., REN X.-R., MILLIGAN G., LEFKOWITZ R.J. β-Arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. - PubMed
-
- BOKOCH G.M., KATADA T., NORTHUP J.K., UI M., GILMAN A.G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem. 1984;259:3560–3567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

