Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone--a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO)
- PMID: 16402269
- PMCID: PMC12161066
- DOI: 10.1007/s00432-005-0074-4
Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone--a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO)
Abstract
Purpose: This randomized phase III study compared bendamustine and prednisone (BP) to standard melphalan and prednisone (MP) treatment in previously untreated patients with multiple Myeloma (MM).
Patients and methods: To be included, patients had to have histologically and cytologically proven stage II with progressive diseases or stage III MM. They were randomly assigned to receive BP (n=68) or MP (n=63). The primary endpoint was the time to treatment failure (TTF). Secondary endpoints included survival, remission rate, toxicity and quality of life.
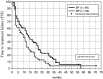
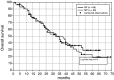
Results: The overall response rate was 75% in the BP and 70% in the MP group. A significantly higher number of patients treated with BP achieved a complete remission than did patients receiving MP (32 vs. 13%; P=0.007), and the maximum response was achieved more rapidly in patients treated with BP compared to those receiving MP (6.8 vs. 8.7 cycles; P<0.02). TTF and remission duration were significantly longer in the BP group. Patients receiving BP had higher QoL scores and reported pain less frequently than patients receiving MP.
Conclusion: BP is superior to MP with respect to complete remission rate, TTF, cycles needed to achieve maximum remission and quality of life and should be considered the new standard in first-line treatment of MM patients not eligible for transplantation.
Figures

References
-
- Alberts DS, Chang SY, Chen HS, Evans TL, Moon TE (1979) Oral melphalan kinetics. Clin Pharmacol Ther 26(6):737–745 - PubMed
-
- Anger G, Hesse P, Baufeld H (1969) Treatment of multiple myeloma with a new cytostatic agent: gamma-l-methyl-5-bis-(beta-chlorethyl)-amino-benzimidazolyl-(2)-butyric acid hydrochloride. DMW 94(48):2495–2500 - PubMed
-
- Anger G, Fink R, Fleischer J, Hesse P, Krug K, Raderecht C et al (1975) Vergleichsuntersuchungen zwischen Cytostasan und Cyclophosphamid bei der chronischen Lymphadenose, dem Plasmozytom, der Lymphogranulomatose und dem Bronchialkarzinom. Dt Gesundh -Wesen 30(27):1280–1285
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

