Synthesis of an artificial cell surface receptor that enables oligohistidine affinity tags to function as metal-dependent cell-penetrating peptides
- PMID: 16402806
- PMCID: PMC2546700
- DOI: 10.1021/ja056126j
Synthesis of an artificial cell surface receptor that enables oligohistidine affinity tags to function as metal-dependent cell-penetrating peptides
Erratum in
- J Am Chem Soc. 2006 Apr 12;128(14):4917
Abstract
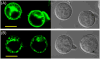
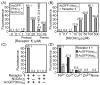
Cell-penetrating peptides and proteins (CPPs) are important tools for the delivery of impermeable molecules into living mammalian cells. To enable these cells to internalize proteins fused to common oligohistidine affinity tags, we synthesized an artificial cell surface receptor comprising an N-alkyl derivative of 3beta-cholesterylamine linked to the metal chelator nitrilotriacetic acid (NTA). This synthetic receptor inserts into cellular plasma membranes, projects NTA headgroups from the cell surface, and rapidly cycles between the plasma membrane and intracellular endosomes. Jurkat lymphocytes treated with the synthetic receptor (10 microM) for 1 h displayed approximately 8,400,000 [corrected]NTA groups on the cell surface. Subsequent addition of the green fluorescent protein AcGFP fused to hexahistidine or decahistidine peptides (3 microM) and Ni(OAc)(2) (100 microM) enhanced the endocytosis of AcGFP by 150-fold (hexahistidine fusion protein) or 600-fold (decahistidine fusion protein) within 4 h at 37 degrees C. No adverse effects on cellular proliferation or morphology were observed under these conditions. By enabling common oligohistidine affinity tags to function as cell-penetrating peptides, this metal-chelating cell surface receptor provides a useful tool for studies of cellular biology [corrected]
Figures


Similar articles
-
Synthetic mimics of small Mammalian cell surface receptors.J Am Chem Soc. 2004 Dec 22;126(50):16379-86. doi: 10.1021/ja046663o. J Am Chem Soc. 2004. PMID: 15600339
-
Selectivity of competitive multivalent interactions at interfaces.Chembiochem. 2009 Jul 20;10(11):1878-87. doi: 10.1002/cbic.200900001. Chembiochem. 2009. PMID: 19565593
-
Double-hexahistidine tag with high-affinity binding for protein immobilization, purification, and detection on ni-nitrilotriacetic acid surfaces.Anal Chem. 2006 May 1;78(9):3072-9. doi: 10.1021/ac060184l. Anal Chem. 2006. PMID: 16642995
-
Synthetic mimics of mammalian cell surface receptors: prosthetic molecules that augment living cells.Org Biomol Chem. 2005 Oct 21;3(20):3607-12. doi: 10.1039/b509866a. Epub 2005 Sep 8. Org Biomol Chem. 2005. PMID: 16211095 Review.
-
Biosensors Based on the Binding Events of Nitrilotriacetic Acid-Metal Complexes.Biosensors (Basel). 2023 Apr 28;13(5):507. doi: 10.3390/bios13050507. Biosensors (Basel). 2023. PMID: 37232868 Free PMC article. Review.
Cited by
-
Assembly of cells and vesicles for organ engineering.Sci Technol Adv Mater. 2011 Oct 10;12(6):064703. doi: 10.1088/1468-6996/12/6/064703. eCollection 2011 Dec. Sci Technol Adv Mater. 2011. PMID: 27877453 Free PMC article. Review.
-
Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials.Nano Today. 2011 Jun 1;6(3):309-325. doi: 10.1016/j.nantod.2011.04.001. Nano Today. 2011. PMID: 21826117 Free PMC article.
-
Application of metal coordination chemistry to explore and manipulate cell biology.Chem Rev. 2009 Oct;109(10):4921-60. doi: 10.1021/cr900134a. Chem Rev. 2009. PMID: 19715312 Free PMC article. Review. No abstract available.
-
Cell-Penetrating Peptides: Applications in Tumor Diagnosis and Therapeutics.Pharmaceutics. 2021 Jun 15;13(6):890. doi: 10.3390/pharmaceutics13060890. Pharmaceutics. 2021. PMID: 34204007 Free PMC article. Review.
-
Synthetic cell surface receptors for delivery of therapeutics and probes.Adv Drug Deliv Rev. 2012 Jun 15;64(9):797-810. doi: 10.1016/j.addr.2012.02.007. Epub 2012 Feb 25. Adv Drug Deliv Rev. 2012. PMID: 22401875 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

