Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo
- PMID: 16402857
- PMCID: PMC1334387
- DOI: 10.1371/journal.pbio.0040020
Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo
Abstract
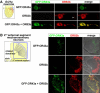
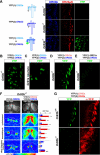
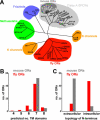
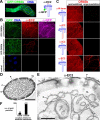
Drosophila olfactory sensory neurons (OSNs) each express two odorant receptors (ORs): a divergent member of the OR family and the highly conserved, broadly expressed receptor OR83b. OR83b is essential for olfaction in vivo and enhances OR function in vitro, but the molecular mechanism by which it acts is unknown. Here we demonstrate that OR83b heterodimerizes with conventional ORs early in the endomembrane system in OSNs, couples these complexes to the conserved ciliary trafficking pathway, and is essential to maintain the OR/OR83b complex within the sensory cilia, where odor signal transduction occurs. The OR/OR83b complex is necessary and sufficient to promote functional reconstitution of odor-evoked signaling in sensory neurons that normally respond only to carbon dioxide. Unexpectedly, unlike all known vertebrate and nematode chemosensory receptors, we find that Drosophila ORs and OR83b adopt a novel membrane topology with their N-termini and the most conserved loops in the cytoplasm. These loops mediate direct association of ORs with OR83b. Our results reveal that OR83b is a universal and integral part of the functional OR in Drosophila. This atypical heteromeric and topological design appears to be an insect-specific solution for odor recognition, making the OR/OR83b complex an attractive target for the development of highly selective insect repellents to disrupt olfactory-mediated host-seeking behaviors of insect disease vectors.
Figures






References
-
- Mombaerts P. Seven-transmembrane proteins as odorant and chemosensory receptors. Science. 1999;286:707–711. - PubMed
-
- van der Goldman AL, Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. - PubMed
-
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. - PubMed
-
- Mombaerts P. Odorant receptor gene choice in olfactory sensory neurons: The one receptor-one neuron hypothesis revisited. Curr Opin Neurobiol. 2004;14:31–36. - PubMed
-
- Barnea G, O'Donnell S, Mancia F, Sun X, Nemes A, et al. Odorant receptors on axon termini in the brain. Science. 2004;304:1468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

