Cytochrome P450 and xenobiotic receptor humanized mice
- PMID: 16402898
- PMCID: PMC1440291
- DOI: 10.1146/annurev.pharmtox.45.120403.100007
Cytochrome P450 and xenobiotic receptor humanized mice
Abstract
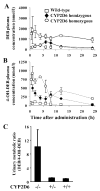
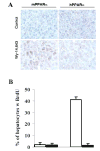
Most xenobiotics that enter the body are subjected to metabolism that functions primarily to facilitate their elimination. Metabolism of certain xenobiotics can also result in the production of electrophilic derivatives that can cause cell toxicity and transformation. Many xenobiotics can also activate receptors that in turn induce the expression of genes encoding xenobiotic-metabolizing enzymes and xenobiotic transporters. However, there are marked species differences in the way mammals respond to xenobiotics, which are due in large part to molecular differences in receptors and xenobiotic-metabolizing enzymes. This presents a problem in extrapolating data obtained with rodent model systems to humans. There are also polymorphisms in xenobiotic-metabolizing enzymes that can impact drug therapy and cancer susceptibility. In an effort to generate more reliable in vivo systems to study and predict human response to xenobiotics, humanized mice are under development.
Figures



References
-
- Gonzalez FJ, Gelboin HV. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev. 1994;26:165–83. - PubMed
-
- Guengerich FP. Cytochromes P450, drugs, and diseases. Mol Interv. 2003;3:194–204. - PubMed
-
- Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. - PubMed
-
- Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–66. - PubMed
-
- Gonzalez FJ, Liu SY, Yano M. Regulation of cytochrome P450 genes: molecular mechanisms. Pharmacogenetics. 1993;3:51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

