Metallothionein isoform 2A expression is inducible and protects against ROS-mediated cell death in rotenone-treated HeLa cells
- PMID: 16402917
- PMCID: PMC1422768
- DOI: 10.1042/BJ20051253
Metallothionein isoform 2A expression is inducible and protects against ROS-mediated cell death in rotenone-treated HeLa cells
Abstract
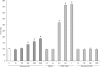
The role of MT (metallothionein) gene expression was investigated in rotenone-treated HeLa cells to induce a deficiency of NADH:ubiquinone oxidoreductase (complex I). Complex I deficiency leads to a diversity of cellular consequences, including production of ROS (reactive oxygen species) and apoptosis. HeLa cells were titrated with rotenone, resulting in dose-dependent decrease in complex I activity and elevated ROS production at activities lower than 33%. Expression of MT2A (MT isoform 2A), but not MT1A or MT1B RNA, was significantly inducible by rotenone (up to 7-fold), t-BHP (t-butyl hydroperoxide; 5-fold) and CdCl2 (50-fold), but not ZnCl2. Myxothiazol treatment did not elevate either ROS or MT2A levels, which supports a ROS-related mechanism for rotenone-induced MT2A expression. To evaluate the role of MT2A expression, MT2A and MT1B were overexpressed in HeLa cells and treated with rotenone. Compared with control and MT1B-overexpressing cells, ROS production was significantly lower and cell viability higher in MT2A-overexpressing HeLa cells when ROS production was enhanced by treatment with t-BHP. Mitochondrial membrane potential was noticeably less reduced in both MT-overexpressing cell lines. MT2A overexpression in rotenone-treated cells also significantly reduced or delayed apoptosis induction, as measured by caspase 3/7 activity and cytosolic nucleosome enrichment. We conclude that MT2A offers significant protection against the main death-causing consequences of rotenone-induced complex I deficiency in HeLa cells. Our results are in support of the protective role against oxidative stress ascribed to MTs and provide evidence that MT2A expression may be a beneficial downstream adaptive response in complex I-deficient cells.
Figures


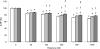
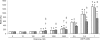
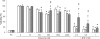


References
-
- Carroll J., Shannon R. J., Fearnley I. M., Walker J. E., Hirst J. Definition of the nuclear encoded protein composition of bovine heart mitochondrial complex I: identification of two new subunits. J. Biol. Chem. 2002;277:50311–50317. - PubMed
-
- Loeffen J. L., Smeitink J. A., Trijbels J. M., Janssen A. J., Triepels R. H., Sengers R. C., van den Heuvel L. P. Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum. Mutat. 2000;15:123–134. - PubMed
-
- Smeitink J. A., van den Heuvel L., DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2001;5:342–352. - PubMed
-
- Barrientos A., Moraes C. T. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J. Biol. Chem. 1999;274:16188–16197. - PubMed
-
- Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J. A., Robinson J. P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

