Two glucose/xylose transporter genes from the yeast Candida intermedia: first molecular characterization of a yeast xylose-H+ symporter
- PMID: 16402921
- PMCID: PMC1462686
- DOI: 10.1042/BJ20051465
Two glucose/xylose transporter genes from the yeast Candida intermedia: first molecular characterization of a yeast xylose-H+ symporter
Abstract
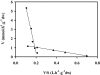
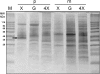
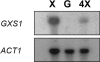
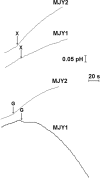
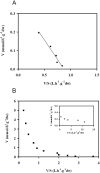
Candida intermedia PYCC 4715 was previously shown to grow well on xylose and to transport this sugar by two different transport systems: high-capacity and low-affinity facilitated diffusion and a high-affinity xylose-proton symporter, both of which accept glucose as a substrate. Here we report the isolation of genes encoding both transporters, designated GXF1 (glucose/xylose facilitator 1) and GXS1 (glucose/xylose symporter 1) respectively. Although GXF1 was isolated by functional complementation of an HXT-null (where Hxt refers to hexose transporters) Saccharomyces cerevisiae strain, isolation of the GXS1 cDNA required partial purification and micro-sequencing of the transporter, identified by its relative abundance in cells grown on low xylose concentrations. Both genes were expressed in S. cerevisiae and the kinetic parameters of glucose and xylose transport were determined. Gxs1 is the first yeast xylose/glucose-H+ symporter to be characterized at the molecular level. Comparison of its amino acid sequence with available sequence data revealed the existence of a family of putative monosaccharide-H+ symporters encompassing proteins from several yeasts and filamentous fungi.
Figures

References
-
- Dien B. S., Cotta M. A., Jeffries T. W. Bacteria engineered for fuel ethanol production: current status. Appl. Microbiol. Biotechnol. 2003;63:258–266. - PubMed
-
- Jeffries T. W., Jin Y. S. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol. 2004;70:495–509. - PubMed
-
- Hahn-Hägerdal B., Hallborn J., Jeppsson H., Olsson L., Skoog K., Walfridsson M. Pentose fermentation to alcohol. In: Saddler J. N., editor. Bioconversion of Forest and Agricultural Plant Residues. Wallingford: CAB International; 1993. pp. 231–290.
-
- Kotyk A. Mobility of the free and of the loaded monosaccharide carrier in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1967;135:112–119. - PubMed
-
- Gárdonyi M., Jeppsson M., Liden G., Gorwa-Grauslund M. F., Hahn-Hägerdal B. Control of xylose consumption by xylose transport in recombinant Saccharomyces cerevisiae. Biotechnol. Bioeng. 2003;82:818–824. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

