Common angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2b
- PMID: 16403216
- PMCID: PMC1360063
- DOI: 10.1186/1742-4682-3-1
Common angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2b
Abstract
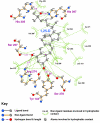
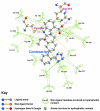
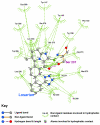
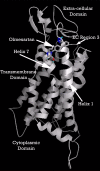
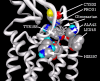
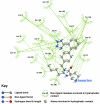
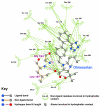
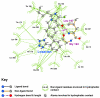
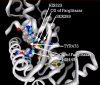
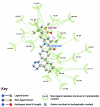
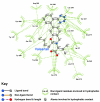
Background: There have been indications that common Angiotensin Receptor Blockers (ARBs) may be exerting anti-inflammatory actions by directly modulating the immune system. We decided to use molecular modelling to rapidly assess which of the potential targets might justify the expense of detailed laboratory validation. We first studied the VDR nuclear receptor, which is activated by the secosteroid hormone 1,25-dihydroxyvitamin-D. This receptor mediates the expression of regulators as ubiquitous as GnRH (Gonadatrophin hormone releasing hormone) and the Parathyroid Hormone (PTH). Additionally we examined Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma), which affects the function of phagocytic cells, and the C-CChemokine Receptor, type 2b, (CCR2b), which recruits monocytes to the site of inflammatory immune challenge.
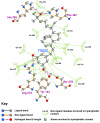
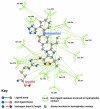
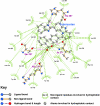
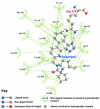
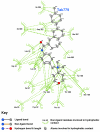
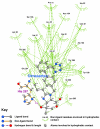
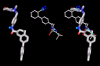
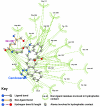
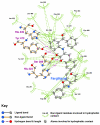
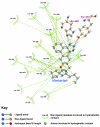
Results: Telmisartan was predicted to strongly antagonize (Ki asymptotically equal to 0.04 nmol) the VDR. The ARBs Olmesartan, Irbesartan and Valsartan (Ki asymptotically equal to10 nmol) are likely to be useful VDR antagonists at typical in-vivo concentrations. Candesartan (Ki asymptotically equal to 30 nmol) and Losartan (Ki asymptotically equal to 70 nmol) may also usefully inhibit the VDR. Telmisartan is a strong modulator of PPARgamma (Ki asymptotically equal to 0.3 nmol), while Losartan (Ki asymptotically equal to 3 nmol), Irbesartan (Ki asymptotically equal to 6 nmol), Olmesartan and Valsartan (Ki asymptotically equal to 12 nmol) also seem likely to have significant PPAR modulatory activity. Olmesartan and Irbesartan (Ki asymptotically equal to 9 nmol) additionally act as antagonists of a theoretical model of CCR2b. Initial validation of this CCR2b model was performed, and a proposed model for the Angiotensin II Type1 receptor (AT2R1) has been presented.
Conclusion: Molecular modeling has proven valuable to generate testable hypotheses concerning receptor/ligand binding and is an important tool in drug design. ARBs were designed to act as antagonists for AT2R1, and it was not surprising to discover their affinity for the structurally similar CCR2b. However, this study also found evidence that ARBs modulate the activation of two key nuclear receptors-VDR and PPARgamma. If our simulations are confirmed by experiment, it is possible that ARBs may become useful as potent anti-inflammatory agents, in addition to their current indication as cardiovascular drugs.
Figures
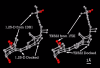
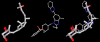
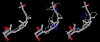

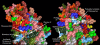

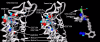

References
-
- United States Food and Drug Administration Approval Package for NDA21-286 http://www.fda.gov/cder/foi/nda/2002/21-286_Benicar_pharmr_P1.pdf Pharmacology Review, figures 1.1.1.1,1.1.1.3 and1.1.1.4.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

