Agroinjection of tomato fruits. A tool for rapid functional analysis of transgenes directly in fruit
- PMID: 16403736
- PMCID: PMC1326026
- DOI: 10.1104/pp.105.068221
Agroinjection of tomato fruits. A tool for rapid functional analysis of transgenes directly in fruit
Abstract
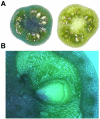
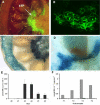
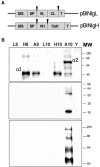
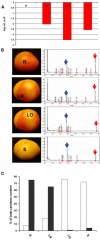
Transient expression of foreign genes in plant tissues is a valuable tool for plant biotechnology. To shorten the time for gene functional analysis in fruits, we developed a transient methodology that could be applied to tomato (Solanum lycopersicum cv Micro Tom) fruits. It was found that injection of Agrobacterium cultures through the fruit stylar apex resulted in complete fruit infiltration. This infiltration method, named fruit agroinjection, rendered high levels of 35S Cauliflower mosaic virus-driven beta-glucuronidase and yellow fluorescence protein transient expression in the fruit, with higher expression levels around the placenta and moderate levels in the pericarp. Usefulness of fruit agroinjection was assayed in three case studies: (1) the heat shock regulation of an Arabidopsis (Arabidopsis thaliana) promoter, (2) the production of recombinant IgA antibodies as an example of molecular farming, and (3) the virus-induced gene silencing of the carotene biosynthesis pathway. In all three instances, this technology was shown to be efficient as a tool for fast transgene expression in fruits.
Figures

Similar articles
-
Cestrum yellow leaf curling virus (CmYLCV) promoter: a new strong constitutive promoter for heterologous gene expression in a wide variety of crops.Plant Mol Biol. 2003 Nov;53(5):663-73. doi: 10.1023/B:PLAN.0000019110.95420.bb. Plant Mol Biol. 2003. PMID: 15010605
-
Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families.Plant J. 2012 May;70(4):585-98. doi: 10.1111/j.1365-313X.2011.04895.x. Epub 2012 Feb 10. Plant J. 2012. PMID: 22211518
-
Isolation and functional characterization of lycopene beta-cyclase (CYC-B) promoter from Solanum habrochaites.BMC Plant Biol. 2010 Apr 9;10:61. doi: 10.1186/1471-2229-10-61. BMC Plant Biol. 2010. PMID: 20380705 Free PMC article.
-
VIGS: a tool to study fruit development in Solanum lycopersicum.Methods Mol Biol. 2013;975:183-96. doi: 10.1007/978-1-62703-278-0_14. Methods Mol Biol. 2013. PMID: 23386304
-
Overaccumulation of higher polyamines in ripening transgenic tomato fruit revives metabolic memory, upregulates anabolism-related genes, and positively impacts nutritional quality.J AOAC Int. 2007 Sep-Oct;90(5):1456-64. J AOAC Int. 2007. PMID: 17955994 Review.
Cited by
-
Isolation and Functional Characterization of a Lycopene β-cyclase Gene Promoter from Citrus.Front Plant Sci. 2016 Sep 13;7:1367. doi: 10.3389/fpls.2016.01367. eCollection 2016. Front Plant Sci. 2016. PMID: 27679644 Free PMC article.
-
Virus-induced gene silencing for functional analysis of selected genes.Plant Cell Rep. 2008 Feb;27(2):209-19. doi: 10.1007/s00299-007-0460-2. Epub 2007 Oct 16. Plant Cell Rep. 2008. Retraction in: Plant Cell Rep. 2009 Feb;28(2):335. doi: 10.1007/s00299-008-0660-4. PMID: 17938933 Retracted. Review.
-
A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis.PLoS One. 2014 Jan 8;9(1):e83556. doi: 10.1371/journal.pone.0083556. eCollection 2014. PLoS One. 2014. PMID: 24416168 Free PMC article.
-
Transient Expression Assay and Microscopic Observation in Kumquat Fruit.Bio Protoc. 2024 Apr 5;14(7):e4968. doi: 10.21769/BioProtoc.4968. eCollection 2024 Apr 5. Bio Protoc. 2024. PMID: 38618180 Free PMC article.
-
Optimisation of the purification process of a tumour-targeting antibody produced in N. benthamiana using vacuum-agroinfiltration.Transgenic Res. 2010 Dec;19(6):1083-97. doi: 10.1007/s11248-010-9382-9. Epub 2010 Mar 15. Transgenic Res. 2010. PMID: 20229286
References
-
- Bentley KJ, Gewert R, Harris WJ (1998) Differential efficiency of expression of humanized antibodies in transient transfected mammalian cells. Hybridoma 17: 559–567 - PubMed
-
- Chen JC, Jiang CZ, Gookin TE, Hunter DA, Clark DG, Reid MS (2004) Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol Biol 55: 521–530 - PubMed
-
- Corthesy B (2002) Recombinant immunoglobulin A: powerful tools for fundamental and applied research. Trends Biotechnol 20: 65–71 - PubMed
-
- D'Aoust MA, Lerouge P, Busse U (2004) Efficient and reliable production of pharmaceuticals in alfalfa. In R Fischer, S Schillberg, eds, Molecular Farming. Wiley-VCH Verlag GmbH & Co., Weinheim, Germany
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

