Acceleration of mesoderm development and expansion of hematopoietic progenitors in differentiating ES cells by the mouse Mix-like homeodomain transcription factor
- PMID: 16403910
- PMCID: PMC1784910
- DOI: 10.1182/blood-2005-10-4120
Acceleration of mesoderm development and expansion of hematopoietic progenitors in differentiating ES cells by the mouse Mix-like homeodomain transcription factor
Abstract
The cellular and molecular events underlying the formation and differentiation of mesoderm to derivatives such as blood are critical to our understanding of the development and function of many tissues and organ systems. How different mesodermal populations are set aside to form specific lineages is not well understood. Although previous genetic studies in the mouse embryo have pointed to a critical role for the homeobox gene Mix-like (mMix) in gastrulation, its function in mesoderm development remains unclear. Hematopoietic defects have been identified in differentiating embryonic stem cells in which mMix was genetically inactivated. Here we show that conditional induction of mMix in embryonic stem cell-derived embryoid bodies results in the early activation of mesodermal markers prior to expression of Brachyury/T and acceleration of the mesodermal developmental program. Strikingly, increased numbers of mesodermal, hemangioblastic, and hematopoietic progenitors form in response to premature activation of mMix. Differentiation to primitive (embryonic) and definitive (adult type) blood cells proceeds normally and without an apparent bias in the representation of different hematopoietic cell fates. Therefore, the mouse Mix gene functions early in the recruitment and/or expansion of mesodermal progenitors to the hemangioblastic and hematopoietic lineages.
Figures


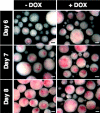

 ) DOX. Markers of the hemangioblast and genes involved in hematopoiesis were activated in response to induced mMix. Expression was normalized to that of a housekeeping gene (for this figure, Gapdh) and expressed as a ratio. For additional details, see Figure 2.
) DOX. Markers of the hemangioblast and genes involved in hematopoiesis were activated in response to induced mMix. Expression was normalized to that of a housekeeping gene (for this figure, Gapdh) and expressed as a ratio. For additional details, see Figure 2.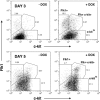
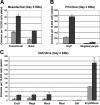
 ) of DOX, harvested on the indicated days, and dispersed to single-cell suspensions. Cells were plated in methylcellulose progenitor assays in triplicate, and colonies were scored as indicated. Colony counts are expressed as mean ± SEM per 10 000 cells plated. (A) Mesodermal progenitors (transitional and blast colonies). EBs were harvested on days 3 and 4 and plated in methylcellulose blast colony cultures. Colonies were scored after 4 days. (B) Primitive erythroid and megakaryocyte colonies. EBs were harvested on day 4 or 5, plated in primitive hematopoietic progenitor cultures, and colonies were scored after 4 or 6 days. (C) Definitive hematopoietic colonies. EBs were harvested on day 4 or 5, plated in Methocult, and scored on day 8 or 10.
) of DOX, harvested on the indicated days, and dispersed to single-cell suspensions. Cells were plated in methylcellulose progenitor assays in triplicate, and colonies were scored as indicated. Colony counts are expressed as mean ± SEM per 10 000 cells plated. (A) Mesodermal progenitors (transitional and blast colonies). EBs were harvested on days 3 and 4 and plated in methylcellulose blast colony cultures. Colonies were scored after 4 days. (B) Primitive erythroid and megakaryocyte colonies. EBs were harvested on day 4 or 5, plated in primitive hematopoietic progenitor cultures, and colonies were scored after 4 or 6 days. (C) Definitive hematopoietic colonies. EBs were harvested on day 4 or 5, plated in Methocult, and scored on day 8 or 10.
Similar articles
-
Mouse Mix gene is activated early during differentiation of ES and F9 stem cells and induces endoderm in frog embryos.Dev Dyn. 2003 Mar;226(3):446-59. doi: 10.1002/dvdy.10263. Dev Dyn. 2003. PMID: 12619131
-
Activin A promotes hematopoietic fated mesoderm development through upregulation of brachyury in human embryonic stem cells.Stem Cells Dev. 2012 Oct 10;21(15):2866-77. doi: 10.1089/scd.2012.0053. Epub 2012 Jun 13. Stem Cells Dev. 2012. PMID: 22548442 Free PMC article.
-
Induction of hematopoietic differentiation of mouse embryonic stem cells by an AGM-derived stromal cell line is not further enhanced by overexpression of HOXB4.Stem Cells Dev. 2010 Nov;19(11):1687-98. doi: 10.1089/scd.2009.0467. Epub 2010 Sep 9. Stem Cells Dev. 2010. PMID: 20184433 Free PMC article.
-
Brachyury, the blastopore and the evolution of the mesoderm.Bioessays. 2001 Sep;23(9):788-94. doi: 10.1002/bies.1114. Bioessays. 2001. PMID: 11536291 Review.
-
Hemangioblast development and regulation.Biochem Cell Biol. 1998;76(6):947-56. Biochem Cell Biol. 1998. PMID: 10392708 Review.
Cited by
-
Correction of sickle cell disease by homologous recombination in embryonic stem cells.Blood. 2006 Aug 15;108(4):1183-8. doi: 10.1182/blood-2006-02-004812. Epub 2006 Apr 25. Blood. 2006. PMID: 16638928 Free PMC article.
-
Molecular and developmental biology of the hemangioblast.Dev Dyn. 2008 May;237(5):1218-31. doi: 10.1002/dvdy.21542. Dev Dyn. 2008. PMID: 18429046 Free PMC article. Review.
-
Lhx2 expression promotes self-renewal of a distinct multipotential hematopoietic progenitor cell in embryonic stem cell-derived embryoid bodies.PLoS One. 2008 Apr 23;3(4):e2025. doi: 10.1371/journal.pone.0002025. PLoS One. 2008. PMID: 18431502 Free PMC article.
-
Mixl1 localizes to putative axial stem cell reservoirs and their posterior descendants in the mouse embryo.Gene Expr Patterns. 2014 May;15(1):8-20. doi: 10.1016/j.gep.2014.02.002. Epub 2014 Mar 12. Gene Expr Patterns. 2014. PMID: 24632399 Free PMC article.
-
Phosphoinositide 3-kinase signalling regulates early development and developmental haemopoiesis.J Cell Sci. 2007 May 15;120(Pt 10):1752-62. doi: 10.1242/jcs.003772. Epub 2007 Apr 24. J Cell Sci. 2007. PMID: 17456549 Free PMC article.
References
-
- Rossant J, Tam PPL. Mouse Development: Patterning, Morphogenesis, and Organogenesis. San Diego: Academic Press; 2002.
-
- Epstein CJ, Erickson RP, Wynshaw-Boris A, eds. Inborn Errors of Development: The Molecular Basis of Clinical Disorders of Morphogenesis. New York, NY: Oxford University Press; 2004.
-
- Tam PL, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68: 3-25. - PubMed
-
- Lu CC, Brennan J, Robertson EJ. From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Develop. 2001;11: 384-392. - PubMed
-
- Tam PP, Gad JM, Kinder SJ, Tsang TE, Behringer RR. Morphogenetic tissue movement and the establishment of body plan during development from blastocyst to gastrula in the mouse. Bioessays. 2001;23: 508-517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

