Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism?
- PMID: 16404427
- PMCID: PMC2361112
- DOI: 10.1038/sj.bjc.6602930
Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism?
Abstract
This study evaluated by immunohistochemistry (IHC) immune cell response during neoadjuvant primary systemic therapy (PST) with trastuzumab in patients with HER2-positive primary breast cancer. In all, 23 patients with IHC 3+ primary breast cancer were treated with trastuzumab plus docetaxel. Pathological complete and partial responses were documented for nine (39%) and 14 (61%) patients, respectively. Case-matched controls comprised patients treated with docetaxel-based PST without trastuzumab (D; n=23) or PST without docetaxel or trastuzumab (non-taxane, non-trastuzumab, NT-NT; n=23). All surgical specimens were blind-analysed by two independent pathologists, with immunohistochemical evaluation of B and T lymphocytes, macrophages, dendritic cells and natural killer (NK) cells. Potential cytolytic cells were stained for Granzyme B and TiA1. HER2 expression was also evaluated in residual tumour cells. Trastuzumab treatment was associated with significantly increased numbers of tumour-associated NK cells and increased lymphocyte expression of Granzyme B and TiA1 compared with controls. This study supports an in vivo role for immune (particularly NK cell) responses in the mechanism of trastuzumab action in breast cancer. These results suggest that trastuzumab plus taxanes lead to enhanced NK cell activity, which may partially account for the synergistic activity of trastuzumab and docetaxel in breast cancer.
Figures
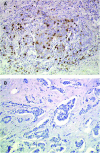

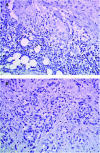

Comment in
-
Administration of anti-HER2 antibody after nonmyeloablative allogeneic stem cell transplantation in metastatic breast cancer.Br J Cancer. 2006 May 22;94(10):1550-2. doi: 10.1038/sj.bjc.6603114. Br J Cancer. 2006. PMID: 16641905 Free PMC article. No abstract available.
References
-
- Baselga J, Gianni L, Geyer C, Perez EA, Riva A, Jackisch C (2004) Future options with trastuzumab for primary systemic and adjuvant therapy. Semin Oncol 31: 51–57 - PubMed
-
- Bines J, Murad A, Lago S, Ferrari B, Andrade J, Filho EA (2003) Weekly docetaxel (Taxotere®) and trastuzumab (Herceptin®) as primary therapy in stage III HER-2 overexpressing breast cancer – a Brazilian multicenter study. Breast Cancer Res Treat 82(Supplement 1): S56 (abstract 243)
-
- Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP (2003) Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol 21: 46–53 - PubMed
-
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23: 3676–3685 - PubMed
-
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA (2001) Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol 31: 3016–3025 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

