IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation
- PMID: 16405428
- PMCID: PMC1450002
- DOI: 10.1042/BJ20052026
IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation
Abstract
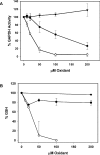
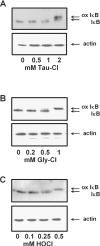
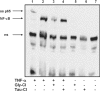
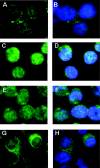
Hypochlorous acid (HOCl) is produced by the neutrophil enzyme, myeloperoxidase, and reacts with amines to generate chloramines. These oxidants react readily with thiols and methionine and can affect cell-regulatory pathways. In the present study, we have investigated the ability of HOCl, glycine chloramine (Gly-Cl) and taurine chloramine (Tau-Cl) to oxidize IkappaBalpha, the inhibitor of NF-kappaB (nuclear factor kappaB), and to prevent activation of the NF-kappaB pathway in Jurkat cells. Glycine chloramine (Gly-Cl) and HOCl were permeable to the cells as determined by oxidation of intracellular GSH and inactivation of glyceraldehyde-3-phosphate dehydrogenase, whereas Tau-Cl showed no detectable cell permeability. Both Gly-Cl (20-200 muM) and HOCl (50 microM) caused oxidation of IkappaBalpha methionine, measured by a shift in electrophoretic mobility, when added to the cells in Hanks buffer. In contrast, a high concentration of Tau-Cl (1 mM) in Hanks buffer had no effect. However, Tau-Cl in full medium did modify IkappaBalpha. This we attribute to chlorine exchange with other amines in the medium to form more permeable chloramines. Oxidation by Gly-Cl prevented IkappaBalpha degradation in cells treated with TNFalpha (tumour necrosis factor alpha) and inhibited nuclear translocation of NF-kappaB. IkappaBalpha modification was reversed by methionine sulphoxide reductase, with both A and B forms required for complete reduction. Oxidized IkappaBalpha persisted intracellularly for up to 6 h. Reversion occurred in the presence of cycloheximide, but was prevented if thioredoxin reductase was inhibited, suggesting that it was due to endogenous methionine sulphoxide reductase activity. These results show that cell-permeable chloramines, either directly or when formed in medium, could regulate NF-kappaB activation via reversible IkappaBalpha oxidation.
Figures




Similar articles
-
Oxidative modification of IkappaB by monochloramine inhibits tumor necrosis factor alpha-induced NF-kappaB activation.Biochim Biophys Acta. 2005 Dec 15;1746(2):135-42. doi: 10.1016/j.bbamcr.2005.10.005. Epub 2005 Oct 28. Biochim Biophys Acta. 2005. PMID: 16344117
-
Chlorine transfer between glycine, taurine, and histamine: reaction rates and impact on cellular reactivity.Free Radic Biol Med. 2004 Nov 15;37(10):1622-30. doi: 10.1016/j.freeradbiomed.2004.08.010. Free Radic Biol Med. 2004. Corrected and republished in: Free Radic Biol Med. 2005 Feb 1;38(3):397-405. doi: 10.1016/j.freeradbiomed.2004.11.006. PMID: 15477013 Corrected and republished.
-
Chlorine transfer between glycine, taurine, and histamine: reaction rates and impact on cellular reactivity.Free Radic Biol Med. 2005 Feb 1;38(3):397-405. doi: 10.1016/j.freeradbiomed.2004.11.006. Free Radic Biol Med. 2005. PMID: 15693173
-
Taurine: new implications for an old amino acid.FEMS Microbiol Lett. 2003 Sep 26;226(2):195-202. doi: 10.1016/S0378-1097(03)00611-6. FEMS Microbiol Lett. 2003. PMID: 14553911 Review.
-
Taurine and its chloramine: modulators of immunity.Neurochem Res. 2004 Jan;29(1):117-26. doi: 10.1023/b:nere.0000010440.37629.17. Neurochem Res. 2004. PMID: 14992270 Review.
Cited by
-
Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1.Antioxid Redox Signal. 2015 Oct 1;23(10):814-22. doi: 10.1089/ars.2015.6385. Epub 2015 Jul 16. Antioxid Redox Signal. 2015. PMID: 26181576 Free PMC article. Review.
-
Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite.Am J Physiol Lung Cell Mol Physiol. 2011 Mar;300(3):L362-9. doi: 10.1152/ajplung.00278.2010. Epub 2010 Dec 10. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21148791 Free PMC article.
-
Halogen-Induced Chemical Injury to the Mammalian Cardiopulmonary Systems.Physiology (Bethesda). 2021 Sep 1;36(5):272-291. doi: 10.1152/physiol.00004.2021. Physiology (Bethesda). 2021. PMID: 34431415 Free PMC article. Review.
-
Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase.Free Radic Res. 2011 Feb;45(2):165-76. doi: 10.3109/10715762.2010.521154. Epub 2010 Oct 18. Free Radic Res. 2011. PMID: 20954832 Free PMC article.
-
Genome-wide impact of hydrogen peroxide on maintenance DNA methylation in replicating cells.Epigenetics Chromatin. 2021 Mar 24;14(1):17. doi: 10.1186/s13072-021-00388-6. Epigenetics Chromatin. 2021. PMID: 33761969 Free PMC article.
References
-
- Baeuerle P. A., Henkel T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 1994;12:141–179. - PubMed
-
- Baeuerle P. A., Baltimore D. IκB: a specific inhibitor of the NF-κB transcription factor. Science. 1988;242:540–546. - PubMed
-
- Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. Control of IκB-α proteolysis by site-specific signal-induced phosphorylation. Science. 1995;267:1485–1488. - PubMed
-
- Henkel T., Machleidt T., Alkalay I., Kronke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature (London) 1993;365:182–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

