Correction of the sickle cell mutation in embryonic stem cells
- PMID: 16407095
- PMCID: PMC1326143
- DOI: 10.1073/pnas.0510177103
Correction of the sickle cell mutation in embryonic stem cells
Abstract
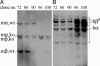
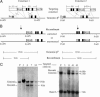
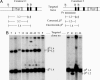
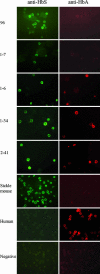
Sickle cell anemia is one of the most common genetic diseases worldwide. Patients often suffer from anemia, painful crises, infections, strokes, and cardiopulmonary complications. Although current management has improved the quality of life and survival of patients, cure can be achieved only with bone marrow transplantation when histocompatible donors are available. The ES cell technology suggests that a therapeutic cloning approach may be feasible for treatment of this disease. Using a transgenic/knockout sickle cell anemia mouse model, which harbors 240 kb of human DNA sequences containing the beta(S)-globin gene, we prepared ES cells from blastocysts that had the sickle cells anemia genotype and carried out homologous recombination with DNA constructs that contained the beta(A)-globin gene. We obtained ES cells in which the beta(S) was corrected to the beta(A) sequence. Hematopoietic cells differentiated from these ES cells produced both hemoglobin A and hemoglobin S. This approach can be applied to human ES cells to correct the sickle mutation as well as beta-thalassemia mutations.
Figures
Similar articles
-
Correction of sickle cell disease by homologous recombination in embryonic stem cells.Blood. 2006 Aug 15;108(4):1183-8. doi: 10.1182/blood-2006-02-004812. Epub 2006 Apr 25. Blood. 2006. PMID: 16638928 Free PMC article.
-
Correction of sickle cell disease in transgenic mouse models by gene therapy.Science. 2001 Dec 14;294(5550):2368-71. doi: 10.1126/science.1065806. Science. 2001. PMID: 11743206
-
Mouse model of human beta zero thalassemia: targeted deletion of the mouse beta maj- and beta min-globin genes in embryonic stem cells.Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9259-63. doi: 10.1073/pnas.92.20.9259. Proc Natl Acad Sci U S A. 1995. PMID: 7568113 Free PMC article.
-
Gene Addition Strategies for β-Thalassemia and Sickle Cell Anemia.Adv Exp Med Biol. 2017;1013:155-176. doi: 10.1007/978-1-4939-7299-9_6. Adv Exp Med Biol. 2017. PMID: 29127680 Free PMC article. Review.
-
Recent advances in globin gene transfer for the treatment of beta-thalassemia and sickle cell anemia.Curr Opin Hematol. 2006 May;13(3):142-8. doi: 10.1097/01.moh.0000219658.57915.d4. Curr Opin Hematol. 2006. PMID: 16567956 Review.
Cited by
-
Use of genome-editing tools to treat sickle cell disease.Hum Genet. 2016 Sep;135(9):1011-28. doi: 10.1007/s00439-016-1688-0. Epub 2016 Jun 1. Hum Genet. 2016. PMID: 27250347 Free PMC article. Review.
-
Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease.Blood. 2011 Oct 27;118(17):4599-608. doi: 10.1182/blood-2011-02-335554. Epub 2011 Aug 31. Blood. 2011. PMID: 21881051 Free PMC article.
-
Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases.Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9826-30. doi: 10.1073/pnas.0904689106. Epub 2009 May 29. Proc Natl Acad Sci U S A. 2009. PMID: 19482945 Free PMC article.
-
Combining Single Strand Oligodeoxynucleotides and CRISPR/Cas9 to Correct Gene Mutations in β-Thalassemia-induced Pluripotent Stem Cells.J Biol Chem. 2016 Aug 5;291(32):16576-85. doi: 10.1074/jbc.M116.719237. Epub 2016 Jun 10. J Biol Chem. 2016. PMID: 27288406 Free PMC article.
-
Gene therapy for hemoglobinopathies: progress and challenges.Transl Res. 2013 Apr;161(4):293-306. doi: 10.1016/j.trsl.2012.12.011. Epub 2013 Jan 19. Transl Res. 2013. PMID: 23337292 Free PMC article. Review.
References
-
- Serjeant, G. R. & Serjeant, B. E. (2001) Sickle Cell Disease (Oxford Univ. Press, Oxford), 3rd Ed.
-
- Beutler, E. (2001) in Williams Hematology, eds. Beutler, E., Lichtman, M. A., Coller, B. S., Kipps, T. J. & Seligsohn, U. (McGraw–Hill, New York), 6th Ed., pp. 584–605.
-
- Sauntharajah, Y., Vichinsky, E. P. & Embury, S. H. (2005) in Hematology: Basic Principle and Practice, eds. Hoffman, R., Benz, E. J., Shattil, S. J., Furie, B., Cohen, H., Silberstein, L. E. & McGlave, P. (Elsevier, Philadephia), pp. 605–644.
-
- Al-Khatti, A., Papayannopoulou, T., Knitter, G., Fritsch, E. F. & Stamatoyannopoulos, G. (1988) Blood 72, 817–819. - PubMed
-
- Veith, R., Galanello, R., Papayannopoulou, T. & Stamatoyannopoulos, G. (1985) N. Engl. J. Med. 313, 1571–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

