Regulation of motor performance and striatal function by synaptic scaffolding proteins of the Homer1 family
- PMID: 16407107
- PMCID: PMC1325014
- DOI: 10.1073/pnas.0505900103
Regulation of motor performance and striatal function by synaptic scaffolding proteins of the Homer1 family
Abstract
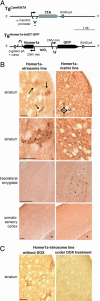
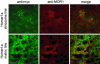
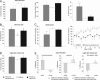
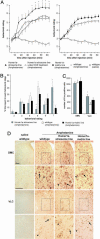
Intracellular calcium mobilization and signaling mechanisms triggered by activation of synaptic glutamate receptors in the striatum are important modulators of neurotransmission in striatal circuits. However, the expression and functions of scaffolding proteins anchoring glutamate receptors at striatal synapses have not been addressed so far. The long-form Homer1 proteins, Homer1b/c, assemble group I metabotropic glutamate receptors (mGluR1/5) in large macromolecular complexes with sources of calcium influx and release at synapses as well as with components of the NMDA receptor complex at the neuronal cell membrane. Homer1a, the short, activity-dependent splice variant of Homer1b/c, lacks the ability of linking mGluR1/5 to synaptic proteins and functions as an endogenous negative modulator of the mGluR1/5 inositol 1,4,5-trisphosphate receptor signaling complex. We have generated transgenic mice, which overexpress Homer1a in striatal medium spiny neurons either homogenously throughout the extrastriosomal matrix (Homer1a-matrix line) or predominantly in striosomal patches (Homer1a-striosome line). Homer1a-expressing mice demonstrated normal development of striatal structure and afferent-efferent connectivity. However, motor performance in behavioral tasks and striatal responses to the psychomotor stimulant amphetamine were significantly altered in the Homer1a-striosome line. Thus, glutamate receptor scaffolding proteins of the Homer1 family critically regulate the functions of striatal medium spiny neurons in complex motor tasks and its modulation by psychomotor stimulant drugs.
Figures
Similar articles
-
In vivo regulation of Homer1a expression in the striatum by cocaine.Mol Pharmacol. 2007 Apr;71(4):1148-58. doi: 10.1124/mol.106.028399. Epub 2007 Jan 18. Mol Pharmacol. 2007. PMID: 17234898
-
Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain.Nat Med. 2006 Jun;12(6):677-81. doi: 10.1038/nm1406. Epub 2006 May 21. Nat Med. 2006. PMID: 16715092
-
Homer1a signaling in the amygdala counteracts pain-related synaptic plasticity, mGluR1 function and pain behaviors.Mol Pain. 2011 May 19;7:38. doi: 10.1186/1744-8069-7-38. Mol Pain. 2011. PMID: 21595930 Free PMC article.
-
The Role of Stress-Induced Changes of Homer1 Expression in Stress Susceptibility.Biochemistry (Mosc). 2021 Jun;86(6):613-626. doi: 10.1134/S0006297921060018. Biochemistry (Mosc). 2021. PMID: 34225586 Review.
-
The Complex Formed by Group I Metabotropic Glutamate Receptor (mGluR) and Homer1a Plays a Central Role in Metaplasticity and Homeostatic Synaptic Scaling.J Neurosci. 2021 Jun 30;41(26):5567-5578. doi: 10.1523/JNEUROSCI.0026-21.2021. J Neurosci. 2021. PMID: 34193623 Free PMC article. Review.
Cited by
-
Identification of Specific Circular RNA Expression Patterns and MicroRNA Interaction Networks in Mesial Temporal Lobe Epilepsy.Front Genet. 2020 Sep 25;11:564301. doi: 10.3389/fgene.2020.564301. eCollection 2020. Front Genet. 2020. PMID: 33101384 Free PMC article.
-
Environmental Enrichment Upregulates Striatal Synaptic Vesicle-Associated Proteins and Improves Motor Function.Front Neurol. 2018 Jul 16;9:465. doi: 10.3389/fneur.2018.00465. eCollection 2018. Front Neurol. 2018. PMID: 30061854 Free PMC article.
-
Severe drug-induced repetitive behaviors and striatal overexpression of VAChT in ChAT-ChR2-EYFP BAC transgenic mice.Front Neural Circuits. 2014 May 28;8:57. doi: 10.3389/fncir.2014.00057. eCollection 2014. Front Neural Circuits. 2014. PMID: 24904300 Free PMC article.
-
Rational and Translational Implications of D-Amino Acids for Treatment-Resistant Schizophrenia: From Neurobiology to the Clinics.Biomolecules. 2022 Jun 29;12(7):909. doi: 10.3390/biom12070909. Biomolecules. 2022. PMID: 35883465 Free PMC article.
-
The neostriatum: two entities, one structure?Brain Struct Funct. 2016 Apr;221(3):1737-49. doi: 10.1007/s00429-015-1000-4. Epub 2015 Feb 5. Brain Struct Funct. 2016. PMID: 25652680 Free PMC article.
References
-
- Bashir, Z. I., Bortolotto, Z. A., Davies, C. H., Berretta, N., Irving, A. J., Seal, A. J., Henley, J. M., Jane, D. E., Watkins, J. C. & Collingridge, G. L. (1993) Nature 363, 347–350. - PubMed
-
- Conquet, F., Bashir, Z. I., Davies, C. H., Daniel, H., Ferraguti, F., Bordi, F., Franz-Bacon, K., Reggiani, A., Matarese, V., Conde, F., et al. (1994) Nature 372, 237–243. - PubMed
-
- Brakeman, P. R., Lanahan, A. A., O'Brien, R., Roche, K., Barnes, C. A., Huganir, R. L. & Worley, P. F. (1997) Nature 368, 284–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

