A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis
- PMID: 16407130
- PMCID: PMC1334658
- DOI: 10.1073/pnas.0508423103
A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis
Abstract
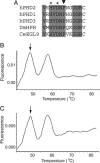
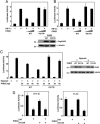
The number of red blood cells is normally tightly regulated by a classic homeostatic mechanism based on oxygen sensing in the kidney. Decreased oxygen delivery resulting from anemia induces the production of erythropoietin, which increases red cell production and hence oxygen delivery. Investigations of erythropoietin regulation identified the transcription factor hypoxia-inducible factor (HIF). HIF is now recognized as being a key regulator of genes that function in a comprehensive range of processes besides erythropoiesis, including energy metabolism and angiogenesis. HIF itself is regulated through the alpha-subunit, which is hydroxylated in the presence of oxygen by a family of three prolyl hydroxylase domain proteins (PHDs)/HIF prolyl hydroxylases/egg-laying-defective nine enzymes. Hydroxylation allows capture by the von Hippel-Lindau tumor suppressor gene product, ubiquitination, and destruction by the proteasome. Here we describe an inherited mutation in a mammalian PHD enzyme. We show that this mutation in PHD2 results in a marked decrease in enzyme activity and is associated with familial erythrocytosis, identifying a previously unrecognized cause of this condition. Our findings indicate that PHD2 is critical for normal regulation of HIF in humans.
Figures

References
-
- Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J. M., Lane, W. S. & Kaelin, W. G., Jr. (2001) Science 292, 464–468. - PubMed
-
- Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., Kriegsheim Av, A., Hebestreit, H. F., Mukherji, M., Schofield, C. J., et al. (2001) Science 292, 468–472. - PubMed
-
- Epstein, A. C., Gleadle, J. M., McNeill, L. A., Hewitson, K. S., O'Rourke, J., Mole, D. R., Mukherji, M., Metzen, E., Wilson, M. I., Dhanda, A., et al. (2001) Cell 107, 43–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

