Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection
- PMID: 16407131
- PMCID: PMC1334656
- DOI: 10.1073/pnas.0507995103
Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection
Abstract
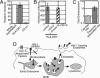
Immature dendritic cells (DCs) capture HIV type 1 (HIV-1) and can transmit captured virus particles to T cells. In this report, we show that HIV-1 particles captured by DCs can be transmitted to T cells by exocytosis without de novo infection. Captured HIV-1 particles were rapidly endocytosed to tetraspan protein (CD9, CD63)-positive endocytic compartments that were reminiscent of multivesicular endosomal bodies. Furthermore, some of the endocytosed virus particles were constitutively released into the extracellular milieu in association with HLA-DR1(+), CD1b(+), CD9(+), and CD63(+) vesicles (exosomes) and could initiate productive infections of CD4(+) target cells. Surprisingly, the exocytosed vesicle-associated HIV-1 particles from DCs were 10-fold more infectious on a perparticle basis than cell-free virus particles. These studies describe a previously undescribed mechanism of DC-mediated HIV-1 transmission and suggest that virus particle trafficking to multivesicular endosomal bodies and subsequent exocytosis can provide HIV-1 particles captured by DCs an avenue for immune escape.
Figures




References
-
- Cameron, P. U., Freudenthal, P. S., Barker, J. M., Gezelter, S., Inaba, K. & Steinman, R. M. (1992) Science 257, 383–387. - PubMed
-
- Geijtenbeek, T. B., Kwon, D. S., Torensma, R., van Vliet, S. J., van Duijnhoven, G. C., Middel, J., Cornelissen, I. L., Nottet, H. S., KewalRamani, V. N., Littman, D. R., et al. (2000) Cell 100, 587–597. - PubMed
-
- Pope, M., Betjes, M. G., Romani, N., Hirmand, H., Cameron, P. U., Hoffman, L., Gezelter, S., Schuler, G. & Steinman, R. M. (1994) Cell 78, 389–398. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

