Mammalian alpha I-spectrin is a neofunctionalized polypeptide adapted to small highly deformable erythrocytes
- PMID: 16407147
- PMCID: PMC1334653
- DOI: 10.1073/pnas.0507661103
Mammalian alpha I-spectrin is a neofunctionalized polypeptide adapted to small highly deformable erythrocytes
Abstract
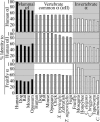
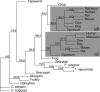
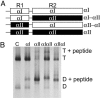
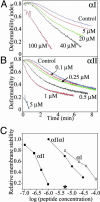
Mammalian red blood cells, unlike those of other vertebrates, must withstand the rigors of circulation in the absence of new protein synthesis. Key to this is plasma membrane elasticity deriving from the protein spectrin, which forms a network on the cytoplasmic face. Spectrin is a tetramer (alphabeta)(2), made up of alphabeta dimers linked head to head. We show here that one component of erythrocyte spectrin, alphaI, is encoded by a gene unique to mammals. Phylogenetic analysis suggests that the other alpha-spectrin gene (alphaII) common to all vertebrates was duplicated after the emergence of amphibia, and that the resulting alphaI gene was preserved only in mammals. The activities of alphaI and alphaII spectrins differ in the context of the human red cell membrane. An alphaI-spectrin fragment containing the site of head-to-head interaction with the beta-chain binds more weakly than the corresponding alphaII fragment to this site. The latter competes so strongly with endogenous alphaI as to cause destabilization of membranes at 100-fold lower concentration than the alphaI fragment. The efficacies of alphaI/alphaII chimeras indicate that the partial structural repeat, which binds to the complementary beta-spectrin element, and the adjacent complete repeat together determine the strength of the dimer-dimer interaction on the membrane. Alignment of all available alpha-spectrin N-terminal sequences reveals three blocks of sequence unique to alphaI. Furthermore, human alphaII-spectrin is closer to fruitfly alpha-spectrin than to human alphaI-spectrin, consistent with adaptation of alphaI to new functions. We conclude that alphaI-spectrin represents a neofunctionalized spectrin adapted to the rapid make and break of tetramers.
Figures


References
-
- Gulliver, G. (1875) Proc. Zool. Soc. London 1875, 474–495.
-
- Hawkey, C. M., Bennett, P. M., Gascoyne, S. C., Hart, M. G. & Kirkwood, J. K. (1991) Br. J. Haematol. 77, 392–397. - PubMed
-
- Gregory, T. R. (2001) Blood Cells Mol. Dis. 27, 830–843. - PubMed
-
- Mohandas, N. & Evans, E. (1994) Annu. Rev. Biophys. Biomol. Struct. 23, 787–818. - PubMed
-
- Discher, D. E. & Carl, P. (2001) Cell Mol. Biol. Lett. 6, 593–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

