Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis
- PMID: 16407152
- PMCID: PMC1334631
- DOI: 10.1073/pnas.0505150103
Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis
Abstract
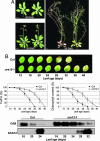
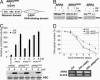
Cytokinins are plant hormones with profound roles in growth and development, including control of leaf longevity. Although the cytokinin signal is known to be perceived by histidine kinase receptors, the underlying molecular mechanism and specificity of the receptors leading to delayed leaf senescence have not yet been elucidated. Here, we found that AHK3, one of the three cytokinin receptors in Arabidopsis, plays a major role in controlling cytokinin-mediated leaf longevity through a specific phosphorylation of a response regulator, ARR2. This result was obtained through identification of a gain-of-function Arabidopsis mutant that shows delayed leaf senescence because of a missense mutation in the extracellular domain of AHK3. A loss-of-function mutation in AHK3, but not of the other cytokinin receptors, conferred a reduced sensitivity to cytokinin in cytokinin-dependent delay of leaf senescence and abolished cytokinin-dependent phosphorylation of ARR2. Consistently, transgenic overexpression of wild-type, but not an unphosphorylatable mutant ARR2, led to delayed senescence of leaves.
Figures




References
-
- Noodén, L. D. (1988) in Senescence and Aging in Plants, eds. Noodén, L. D. & Leopold, A. C. (Academic, San Diego), pp. 1–50.
-
- Nam, H. G. (1997) Curr. Opin. Biotech. 8, 200–207. - PubMed
-
- Gut, H., Ruts, C., Matile, P. & Thomas, H. (1987) Physiol. Plant. 70, 659–663.
-
- Himelblau, E. & Amasino, M. (2001) J. Exp. Bot. 158, 1317–1323.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

