Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons
- PMID: 16407156
- PMCID: PMC1326162
- DOI: 10.1073/pnas.0507225103
Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons
Abstract
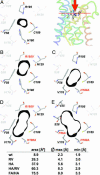
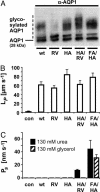
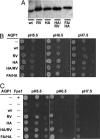
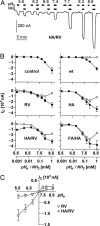
Water-specific aquaporins (AQP), such as the prototypical mammalian AQP1, stringently exclude the passage of solutes, ions, and even protons. Supposedly, this is accomplished by two conserved regions within the pore, a pair of canonical asparagine-proline-alanine (NPA) motifs, the central constriction, and an aromatic/arginine (ar/R) constriction, the outer constriction. Here, we analyzed the function of three residues in the ar/R constriction (Phe-56, His-180, and Arg-195) in rat AQP1. Individual or joint replacement of His-180 and Arg-195 by alanine and valine residues, respectively (AQP1-H180A, AQP1-R195V, and AQP1-H180A/R195V), did not affect water permeability. The double mutant AQP1-H180A/R195V allowed urea to pass. In line with the predicted solute discrimination by size, replacement of both Phe-56 and His-180 (AQP1-F56A/H180A) enlarged the maximal diameter of the ar/R constriction 3-fold and enabled glycerol and urea to pass. We further show that ammonia passes through all four AQP1 mutants, as determined (i) by growth complementation of yeast deletion strains with ammonia, (ii) by ammonia uptake from the external solution into oocytes, and (iii) by direct recordings of ammonia induced proton currents in oocytes. Unexpectedly, removal of the positive charge in the ar/R constriction in AQP1-R195V and AQP1-H180A/R195V appeared to allow the passage of protons through AQP1. The data indicate that the ar/R constriction is a major checkpoint for solute permeability, and that the exquisite electrostatic proton barrier in AQPs comprises both the NPA constriction as well as the ar/R constriction.
Figures
Similar articles
-
The arginine-facing amino acid residue of the rat aquaporin 1 constriction determines solute selectivity according to its size and lipophilicity.Mol Membr Biol. 2014 Nov-Dec;31(7-8):228-38. doi: 10.3109/09687688.2014.960493. Epub 2014 Oct 24. Mol Membr Biol. 2014. PMID: 25341953
-
In vitro analysis and modification of aquaporin pore selectivity.Handb Exp Pharmacol. 2009;(190):77-92. doi: 10.1007/978-3-540-79885-9_4. Handb Exp Pharmacol. 2009. PMID: 19096773 Review.
-
Enhancement of proton conductance by mutations of the selectivity filter of aquaporin-1.J Mol Biol. 2011 Apr 8;407(4):607-20. doi: 10.1016/j.jmb.2011.01.036. Epub 2011 Jan 28. J Mol Biol. 2011. PMID: 21277313
-
Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion.FEBS J. 2011 Mar;278(5):740-8. doi: 10.1111/j.1742-4658.2010.07993.x. Epub 2011 Jan 12. FEBS J. 2011. PMID: 21205205
-
Ammonia and urea permeability of mammalian aquaporins.Handb Exp Pharmacol. 2009;(190):327-58. doi: 10.1007/978-3-540-79885-9_17. Handb Exp Pharmacol. 2009. PMID: 19096786 Review.
Cited by
-
Charge delocalization in proton channels, I: the aquaporin channels and proton blockage.Biophys J. 2007 Jan 1;92(1):46-60. doi: 10.1529/biophysj.106.091934. Epub 2006 Oct 20. Biophys J. 2007. PMID: 17056733 Free PMC article.
-
Identification of Ixodid Tick-Specific Aquaporin-1 Potential Anti-tick Vaccine Epitopes: An in-silico Analysis.Front Bioeng Biotechnol. 2019 Sep 26;7:236. doi: 10.3389/fbioe.2019.00236. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31612130 Free PMC article.
-
Increasing Salt Rejection of Polybenzimidazole Nanofiltration Membranes via the Addition of Immobilized and Aligned Aquaporins.Processes (Basel). 2019 Feb;7(2):76. doi: 10.3390/pr7020076. Epub 2019 Feb 3. Processes (Basel). 2019. PMID: 31179235 Free PMC article.
-
Water flux through human aquaporin 1: inhibition by intracellular furosemide and maximal response with high osmotic gradients.Eur Biophys J. 2011 Jun;40(6):737-46. doi: 10.1007/s00249-011-0687-2. Epub 2011 Mar 4. Eur Biophys J. 2011. PMID: 21373963
-
Organ-specific splice variants of aquaporin water channel AgAQP1 in the malaria vector Anopheles gambiae.PLoS One. 2013 Sep 16;8(9):e75888. doi: 10.1371/journal.pone.0075888. eCollection 2013. PLoS One. 2013. PMID: 24066188 Free PMC article.
References
-
- Zardoya, R. (2005) Biol. Cell 97, 397-414. - PubMed
-
- Walz, T., Hirai, T., Murata, K., Heymann, J. B., Mitsuoka, K., Fujiyoshi, Y., Smith, B. L., Agre, P. & Engel, A. (1997) Nature 387, 624-627. - PubMed
-
- Cheng, A., van Hoek, A. N., Yeager, M., Verkman, A. S. & Mitra, A. K. (1997) Nature 387, 627-630. - PubMed
-
- Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, J. B., Engel, A. & Fujiyoshi, Y. (2001) Nature 407, 599-605. - PubMed
-
- Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. (2001) Nature 414, 872-878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

