The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site
- PMID: 16407162
- PMCID: PMC1326157
- DOI: 10.1073/pnas.0506618103
The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site
Abstract
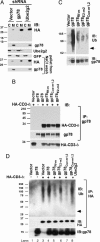
Efficient targeting of proteins for degradation from the secretory pathway is essential to homeostasis. This occurs through endoplasmic reticulum (ER)-associated degradation (ERAD). In this study, we establish that a human ubiquitin ligase (E3), gp78, and a specific E2, Ube2g2, are both critically important for ERAD of multiple substrates. gp78 exhibits a complex domain structure that, in addition to the RING finger, includes a ubiquitin-binding Cue domain and a specific binding site for Ube2g2. Disruption of either of these domains abolishes gp78-mediated ubiquitylation and protein degradation, resulting in accumulation of substrates in their fully glycosylated forms in the ER. This suggests that gp78-mediated ubiquitylation is an early step in ERAD that precedes dislocation of substrates from the ER. The in vivo requirement for both an E2-binding site distinct from the RING finger and a ubiquitin-binding domain intrinsic to an E3 suggests a previously unappreciated level of complexity in ubiquitin ligase function. These results also provide proof of principle that interrupting a specific E2-E3 interaction can selectively inhibit ERAD.
Figures



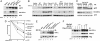
References
-
- Hampton, R. Y. (2002) Curr. Opin. Cell Biol. 14, 476-482. - PubMed
-
- Jarosch, E., Lenk, U. & Sommer, T. (2003) Int. Rev. Cytol. 223, 39-81. - PubMed
-
- Bays, N. W., Gardner, R. G., Seelig, L. P., Joazeiro, C. A. & Hampton, R. Y. (2001) Nat. Cell Biol. 3, 24-29. - PubMed
-
- Deak, P. M. & Wolf, D. H. (2001) J. Biol. Chem. 276, 10663-11069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

