Top-down facilitation of visual recognition
- PMID: 16407167
- PMCID: PMC1326160
- DOI: 10.1073/pnas.0507062103
Top-down facilitation of visual recognition
Erratum in
- Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):3007. Schmidt, AM [corrected to Schmid, AM]
Abstract
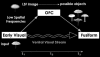
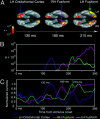
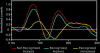
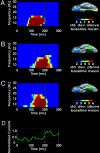
Cortical analysis related to visual object recognition is traditionally thought to propagate serially along a bottom-up hierarchy of ventral areas. Recent proposals gradually promote the role of top-down processing in recognition, but how such facilitation is triggered remains a puzzle. We tested a specific model, proposing that low spatial frequencies facilitate visual object recognition by initiating top-down processes projected from orbitofrontal to visual cortex. The present study combined magnetoencephalography, which has superior temporal resolution, functional magnetic resonance imaging, and a behavioral task that yields successful recognition with stimulus repetitions. Object recognition elicited differential activity that developed in the left orbitofrontal cortex 50 ms earlier than it did in recognition-related areas in the temporal cortex. This early orbitofrontal activity was directly modulated by the presence of low spatial frequencies in the image. Taken together, the dynamics we revealed provide strong support for the proposal of how top-down facilitation of object recognition is initiated, and our observations are used to derive predictions for future research.
Figures



References
-
- Kosslyn, S. M. (1994) Image and Brain (MIT Press, Cambridge, MA).
-
- Ullman, S. (1995) Cereb. Cortex 1, 1-11. - PubMed
-
- Grossberg, S. (1980) Psychol. Rev. 87, 1-51. - PubMed
-
- Bullier, J. (2001) Brain Res. Rev. 36, 96-107. - PubMed
-
- Engel, A. K., Fries, P. & Singer, W. (2001) Nat. Rev. Neurosci. 2, 704-716. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

