APOBEC3A and APOBEC3B are potent inhibitors of LTR-retrotransposon function in human cells
- PMID: 16407327
- PMCID: PMC1326241
- DOI: 10.1093/nar/gkj416
APOBEC3A and APOBEC3B are potent inhibitors of LTR-retrotransposon function in human cells
Abstract
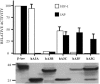
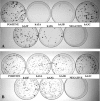
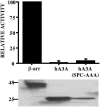
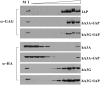
While the ability of APOBEC3G to reduce the replication of a range of exogenous retroviruses is now well established, recent evidence has suggested that APOBEC3G can also inhibit the replication of endogenous retrotransposons that bear long terminal repeats. Here, we extend this earlier work by showing that two other members of the human APOBEC3 protein family, APOBEC3B and APOBEC3A, can reduce retrotransposition by the intracisternal A-particle (IAP) retrotransposon in human cells by 20-fold to up to 100-fold, respectively. This compares to an approximately 4-fold inhibition in IAP retrotransposition induced by APOBEC3G. While both APOBEC3G and APOBEC3B specifically interact with the IAP Gag protein in co-expressing cells, and induce extensive editing of IAP reverse transcripts, APOBEC3A fails to package detectably into IAP virus-like particles and does not edit IAP reverse transcripts. These data, which identify human APOBEC3A as a highly potent inhibitor of LTR-retrotransposon function, are the first to ascribe a biological activity to APOBEC3A. Moreover, these results argue that APOBEC3A inhibits IAP retrotransposition via a novel mechanism that is distinct from, and in this case more effective than, the DNA editing mechanism characteristic of APOBEC3G and APOBEC3B.
Figures

References
-
- Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. - PubMed
-
- Liddament M.T., Brown W.L., Schumacher A.J., Harris R.S. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr. Biol. 2004;14:1385–1391. - PubMed
-
- Bishop K.N., Holmes R.K., Sheehy A.M., Davidson N.O., Cho S.-J., Malim M.H. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 2004;14:1392–1396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

