Role of LrpC from Bacillus subtilis in DNA transactions during DNA repair and recombination
- PMID: 16407330
- PMCID: PMC1326243
- DOI: 10.1093/nar/gkj418
Role of LrpC from Bacillus subtilis in DNA transactions during DNA repair and recombination
Abstract
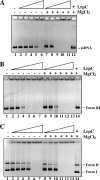
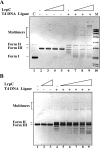
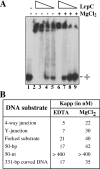
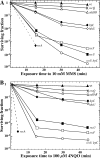
Bacillus subtilis LrpC is a sequence-independent DNA-binding and DNA-bending protein, which binds both single-stranded (ss) and double-stranded (ds) DNA and facilitates the formation of higher order protein-DNA complexes in vitro. LrpC binds at different sites within the same DNA molecule promoting intramolecular ligation. When bound to separate molecules, it promotes intermolecular ligation, and joint molecule formation between a circular ssDNA and a homologous ssDNA-tailed linear dsDNA. LrpC binding showed a higher affinity for 4-way (Holliday) junctions in their open conformation, when compared with curved dsDNA. Consistent with these biochemical activities, an lrpC null mutant strain rendered cells sensitive to DNA damaging agents such as methyl methanesulfonate and 4-nitroquinoline-1-oxide, and showed a segregation defect. These findings collectively suggest that LrpC may be involved in DNA transactions during DNA repair and recombination.
Figures


Similar articles
-
Bacillus subtilis RecU protein cleaves Holliday junctions and anneals single-stranded DNA.Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):452-7. doi: 10.1073/pnas.2533829100. Epub 2003 Dec 30. Proc Natl Acad Sci U S A. 2004. PMID: 14701911 Free PMC article.
-
A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA.Cell. 2007 Sep 7;130(5):824-36. doi: 10.1016/j.cell.2007.07.038. Cell. 2007. PMID: 17803906
-
Bacillus subtilis LrpC is a sequence-independent DNA-binding and DNA-bending protein which bridges DNA.Nucleic Acids Res. 2000 Jan 15;28(2):552-9. doi: 10.1093/nar/28.2.552. Nucleic Acids Res. 2000. PMID: 10606655 Free PMC article.
-
Early steps of double-strand break repair in Bacillus subtilis.DNA Repair (Amst). 2013 Mar 1;12(3):162-76. doi: 10.1016/j.dnarep.2012.12.005. Epub 2013 Feb 4. DNA Repair (Amst). 2013. PMID: 23380520 Review.
-
Nucleoid organization and the maintenance of DNA integrity in E. coli, B. subtilis and D. radiodurans.J Struct Biol. 2006 Nov;156(2):311-9. doi: 10.1016/j.jsb.2006.05.014. Epub 2006 Jul 8. J Struct Biol. 2006. PMID: 16935006 Review.
Cited by
-
Systematic engineering of branch chain amino acid supply modules for the enhanced production of bacitracin from Bacillus licheniformis.Metab Eng Commun. 2020 Jun 11;11:e00136. doi: 10.1016/j.mec.2020.e00136. eCollection 2020 Dec. Metab Eng Commun. 2020. PMID: 32637317 Free PMC article.
-
Short-chain chromate ion transporter proteins from Bacillus subtilis confer chromate resistance in Escherichia coli.J Bacteriol. 2009 Sep;191(17):5441-5. doi: 10.1128/JB.00625-09. Epub 2009 Jul 6. J Bacteriol. 2009. PMID: 19581367 Free PMC article.
-
Enhancement of precursor amino acid supplies for improving bacitracin production by activation of branched chain amino acid transporter BrnQ and deletion of its regulator gene lrp in Bacillus licheniformis.Synth Syst Biotechnol. 2018 Nov 2;3(4):236-243. doi: 10.1016/j.synbio.2018.10.009. eCollection 2018 Dec. Synth Syst Biotechnol. 2018. PMID: 30417137 Free PMC article.
-
Pleiotropic Clostridioides difficile Cyclophilin PpiB Controls Cysteine-Tolerance, Toxin Production, the Central Metabolism and Multiple Stress Responses.Front Pharmacol. 2019 Apr 5;10:340. doi: 10.3389/fphar.2019.00340. eCollection 2019. Front Pharmacol. 2019. PMID: 31024308 Free PMC article.
-
Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells.PLoS Genet. 2009 Sep;5(9):e1000630. doi: 10.1371/journal.pgen.1000630. Epub 2009 Sep 4. PLoS Genet. 2009. PMID: 19730681 Free PMC article.
References
-
- Brinkman A.B., Ettema T.J., De Vos W.M., Van Der Oost J. The Lrp family of transcriptional regulators. Mol. Microbiol. 2003;48:287–294. - PubMed
-
- Newman E.B., Lin R. Leucine-responsive regulatory protein: a global regulator of gene expression in Escherichia coli. Annu. Rev. Microbiol. 1995;49:747–775. - PubMed
-
- Hung S.P., Baldi P., Hatfield G.W. Global gene expression profiling in Escherichia coli K12. The effects of leucine-responsive regulatory protein. J. Biol. Chem. 2002;277:40309–40323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

