Conditional dominant mutations in the Caenorhabditis elegans gene act-2 identify cytoplasmic and muscle roles for a redundant actin isoform
- PMID: 16407404
- PMCID: PMC1382297
- DOI: 10.1091/mbc.e05-09-0886
Conditional dominant mutations in the Caenorhabditis elegans gene act-2 identify cytoplasmic and muscle roles for a redundant actin isoform
Abstract
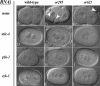
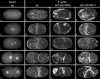
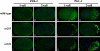
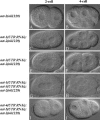
Animal genomes each encode multiple highly conserved actin isoforms that polymerize to form the microfilament cytoskeleton. Previous studies of vertebrates and invertebrates have shown that many actin isoforms are restricted to either nonmuscle (cytoplasmic) functions, or to myofibril force generation in muscle cells. We have identified two temperature-sensitive and semidominant embryonic-lethal Caenorhabditis elegans mutants, each with a single mis-sense mutation in act-2, one of five C. elegans genes that encode actin isoforms. These mutations alter conserved and adjacent amino acids predicted to form part of the ATP binding pocket of actin. At the restrictive temperature, both mutations resulted in aberrant distributions of cortical microfilaments associated with abnormal and striking membrane ingressions and protrusions. In contrast to the defects caused by these dominant mis-sense mutations, an act-2 deletion did not result in early embryonic cell division defects, suggesting that additional and redundant actin isoforms are involved. Accordingly, we found that two additional actin isoforms, act-1 and act-3, were required redundantly with act-2 for cytoplasmic function in early embryonic cells. The act-1 and -3 genes also have been implicated previously in muscle function. We found that an ACT-2::GFP reporter was expressed cytoplasmically in embryonic cells and also was incorporated into contractile filaments in adult muscle cells. Furthermore, one of the dominant act-2 mutations resulted in uncoordinated adult movement. We conclude that redundant C. elegans actin isoforms function in both muscle and nonmuscle contractile processes.
Figures


Similar articles
-
Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans.Anat Rec (Hoboken). 2014 Sep;297(9):1548-59. doi: 10.1002/ar.22965. Anat Rec (Hoboken). 2014. PMID: 25125169 Free PMC article. Review.
-
Caenorhabditis elegans UNC-96 is a new component of M-lines that interacts with UNC-98 and paramyosin and is required in adult muscle for assembly and/or maintenance of thick filaments.Mol Biol Cell. 2006 Sep;17(9):3832-47. doi: 10.1091/mbc.e06-02-0144. Epub 2006 Jun 21. Mol Biol Cell. 2006. PMID: 16790495 Free PMC article.
-
The two actin-interacting protein 1 genes have overlapping and essential function for embryonic development in Caenorhabditis elegans.Mol Biol Cell. 2011 Jul 1;22(13):2258-69. doi: 10.1091/mbc.E10-12-0934. Epub 2011 May 5. Mol Biol Cell. 2011. PMID: 21551072 Free PMC article.
-
The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments.J Cell Biol. 2001 Mar 19;152(6):1313-9. doi: 10.1083/jcb.152.6.1313. J Cell Biol. 2001. PMID: 11257131 Free PMC article.
-
Roles of Actin in the Morphogenesis of the Early Caenorhabditis elegans Embryo.Int J Mol Sci. 2020 May 21;21(10):3652. doi: 10.3390/ijms21103652. Int J Mol Sci. 2020. PMID: 32455793 Free PMC article. Review.
Cited by
-
Smooth muscle α actin is specifically required for the maintenance of lactation.Dev Biol. 2012 Mar 1;363(1):1-14. doi: 10.1016/j.ydbio.2011.11.002. Epub 2011 Nov 12. Dev Biol. 2012. PMID: 22123032 Free PMC article.
-
Branched-chain actin dynamics polarizes vesicle trajectories and partitions apicobasal epithelial membrane domains.Sci Adv. 2023 Jun 28;9(26):eade4022. doi: 10.1126/sciadv.ade4022. Epub 2023 Jun 28. Sci Adv. 2023. PMID: 37379384 Free PMC article.
-
Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans.Anat Rec (Hoboken). 2014 Sep;297(9):1548-59. doi: 10.1002/ar.22965. Anat Rec (Hoboken). 2014. PMID: 25125169 Free PMC article. Review.
-
Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization.Nat Cell Biol. 2017 Aug;19(8):988-995. doi: 10.1038/ncb3577. Epub 2017 Jul 24. Nat Cell Biol. 2017. PMID: 28737772
-
Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy.Nat Cell Biol. 2013 Jan;15(1):103-12. doi: 10.1038/ncb2639. Epub 2012 Dec 16. Nat Cell Biol. 2013. PMID: 23242217 Free PMC article.
References
-
- Albertson, D. G. (1984). Formation of the first cleavage spindle in nematode embryos. Dev. Biol. 101, 61–72. - PubMed
-
- Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., and Struhl, K. (1991). Current Protocols in Molecular Biology, New York: Greene Publishing Associates and Wiley-Interscience.
-
- Bowerman, B., Eaton, B. A., and Priess, J. R. (1992). skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell 68, 1061–1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

