Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance
- PMID: 16407438
- PMCID: PMC1361334
- DOI: 10.1104/pp.105.074906
Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance
Abstract
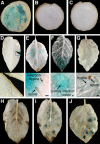
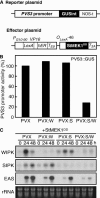
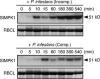
Late blight, caused by the notorious pathogen Phytophthora infestans, is a devastating disease of potato (Solanum tuberosum) and tomato (Solanum lycopersicum), and during the 1840s caused the Irish potato famine and over one million fatalities. Currently, grown potato cultivars lack adequate blight tolerance. Earlier cultivars bred for resistance used disease resistance genes that confer immunity only to some strains of the pathogen harboring corresponding avirulence gene. Specific resistance gene-mediated immunity and chemical controls are rapidly overcome in the field when new pathogen races arise through mutation, recombination, or migration from elsewhere. A mitogen-activated protein kinase (MAPK) cascade plays a pivotal role in plant innate immunity. Here we show that the transgenic potato plants that carry a constitutively active form of MAPK kinase driven by a pathogen-inducible promoter of potato showed high resistance to early blight pathogen Alternaria solani as well as P. infestans. The pathogen attack provoked defense-related MAPK activation followed by induction of NADPH oxidase gene expression, which is implicated in reactive oxygen species production, and resulted in hypersensitive response-like phenotype. We propose that enhancing disease resistance through altered regulation of plant defense mechanisms should be more durable and publicly acceptable than engineering overexpression of antimicrobial proteins.
Figures





References
-
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 - PubMed
-
- Back K, Chappell J (1995) Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus muticus and its molecular comparison to related terpene cyclases. J Biol Chem 270: 7375–7381 - PubMed
-
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

