Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco
- PMID: 16407450
- PMCID: PMC1361315
- DOI: 10.1104/pp.105.071910
Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco
Abstract
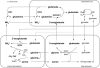
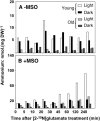
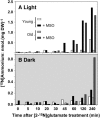
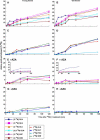
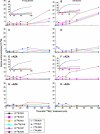
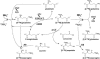
Glutamate (Glu) metabolism and amino acid translocation were investigated in the young and old leaves of tobacco (Nicotiana tabacum L. cv Xanthi) using [15N]ammonium and [2-15N]Glu tracers. Regardless of leaf age, [15N]ammonium assimilation occurred via glutamine synthetase (GS; EC 6.1.1.3) and Glu synthase (ferredoxin [Fd]-GOGAT; EC 1.4.7.1; NADH-GOGAT; EC 1.4.1.14), both in the light and darkness, and it did not depend on Glu dehydrogenase (GDH; EC 1.4.1.2). The [15N]ammonium and ammonium accumulation patterns support the role of GDH in the deamination of [2-15N]Glu to provide 2-oxoglutarate and [15N]ammonium. In the dark, excess [15N]ammonium was incorporated into asparagine that served as an additional detoxification molecule. The constant Glu levels in the phloem sap suggested that Glu was continuously synthesized and supplied into the phloem regardless of leaf age. Further study using transgenic tobacco lines, harboring the promoter of the GLU1 gene (encoding Arabidopsis [Arabidopsis thaliana] Fd-GOGAT) fused to a GUS reporter gene, revealed that the expression of Fd-GOGAT remained higher in young leaves compared to old leaves, and higher in the veins compared to the mesophyll. Confocal laser-scanning microscopy localized the Fd-GOGAT protein to the phloem companion cells-sieve element complex in the leaf veins. The results are consistent with a role of Fd-GOGAT in supplying Glu for the synthesis and transport of amino acids. Taken together, the data provide evidence that the GS-GOGAT pathway and GDH play distinct roles in the source-sink nitrogen cycle of tobacco leaves.
Figures


References
-
- Atkins C (2000) Biochemical aspects of assimilate transfers along the phloem path: N solutes in lupin. Aust J Plant Physiol 27: 531–537
-
- Aubert S, Bligny R, Douce R, Gout E, Ratcliffe RG, Roberts JK (2001) Contribution of glutamate dehydrogenase to mitochondrial glutamate metabolism studied by 13C and 31P nuclear magnetic resonance. J Exp Bot 52: 37–45 - PubMed
-
- Blackwell RD, Murray AJS, Lea PJ, Joy KW (1988) Photorespiratory amino donors, sucrose synthesis and the induction of CO2 fixation in barley deficient in glutamine synthetase and/or glutamate synthase. J Exp Bot 39: 845–858
-
- Bradford MM (1976) A rapid and sensitive method for utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

