Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine
- PMID: 16407535
- PMCID: PMC6674410
- DOI: 10.1523/JNEUROSCI.2761-05.2006
Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine
Abstract
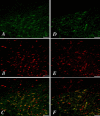
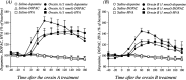
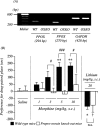
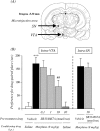
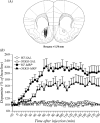
In this study, we investigated the role of orexinergic systems in dopamine-related behaviors induced by the mu-opioid receptor agonist morphine in rodents. Extensive coexpression of tyrosine hydroxylase with orexin receptors was observed in the mouse ventral tegmental area (VTA). The levels of dopamine and its major metabolites in the nucleus accumbens were markedly increased by the microinjection of orexin A and orexin B into the VTA. The subcutaneous morphine-induced place preference and hyperlocomotion observed in wild-type mice were abolished in mice that lacked the prepro-orexin gene. An intra-VTA injection of a selective orexin receptor antagonist SB334867A [1-(2-methylbenzoxazol-6-yl)-3-[1.5]naphthyridin-4-yl urea] significantly suppressed the morphine-induced place preference in rats. Furthermore, the increased level of dialysate dopamine produced by morphine in the mouse brain was significantly decreased by deletion of the prepro-orexin gene. These findings provide new evidence that orexin-containing neurons in the VTA are directly implicated in the rewarding effect and hyperlocomotion induced by morphine through activation of the mesolimbic dopamine pathway in rodents.
Figures


References
-
- Alhaider AA (1991) Antinociceptive effect of ketanserin in mice: involvement of supraspinal 5-HT2 receptors in nociceptive transmission. Brain Res 543: 335–340. - PubMed
-
- Aoki T, Narita M, Ohnishi O, Mizuo K, Narita M, Yajima Y, Suzuki T (2003) Disruption of the type 1 inositol 1,4,5-trisphosphate receptor gene suppresses the morphine-induced antinociception in the mouse. Neurosci Lett 350: 69–72. - PubMed
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993) Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495. - PubMed
-
- Berridge KC (1996) Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25. - PubMed
-
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28: 309–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
