Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2
- PMID: 16407541
- PMCID: PMC6674390
- DOI: 10.1523/JNEUROSCI.2875-05.2006
Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2
Abstract
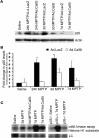
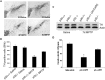
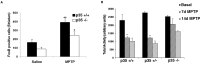
The mechanisms underlying dopamine neuron loss in Parkinson's disease (PD) are not clearly defined. Here, we delineate a pathway by which dopaminergic loss induced by 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine (MPTP) is controlled in vivo. We reported previously that calpains play a central required role in dopamine loss after MPTP treatment. Here, we provide evidence that the downstream effector pathway of calpains is through cyclin-dependent kinase 5 (cdk5)-mediated modulation of the transcription factor myocyte enhancer factor 2 (MEF2). We show that MPTP-induced conversion of the cdk5 activator p35 to a pathogenic p25 form is dependent on calpain activity in vivo. In addition, p35 deficiency attenuates MPTP-induced dopamine neuron loss and behavioral outcome. Moreover, MEF2 is phosphorylated on Ser444, an inactivating site, after MPTP treatment. This phosphorylation is dependent on both calpain and p35 activity, consistent with the model that calpain-mediated activation of cdk5 results in phosphorylation of MEF2 in vivo. Finally, we provide evidence that MEF2 is critical for dopaminergic loss because "cdk5 phosphorylation site mutant" of MEF2D provides neuroprotection in an MPTP mouse model of PD. Together, these data indicate that calpain-p35-p25/cdk5-mediated inactivation of MEF2 plays a critical role in dopaminergic loss in vivo.
Figures


References
-
- Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247. - PubMed
-
- Carafoli E, Molinari M (1998) Calpain: a protease in search of a function? Biochem Biophys Res Commun 247: 193–203. - PubMed
-
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18: 29–42. - PubMed
-
- Chan SL, Mattson MP (1999) Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res 58: 167–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
