Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer's disease and dementia with Lewy bodies
- PMID: 16407544
- PMCID: PMC6674412
- DOI: 10.1523/JNEUROSCI.4265-05.2006
Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer's disease and dementia with Lewy bodies
Abstract
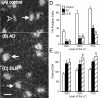
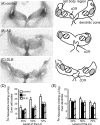
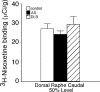
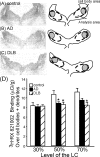
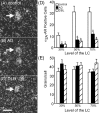
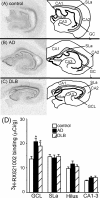
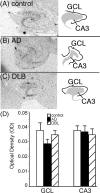
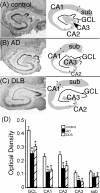
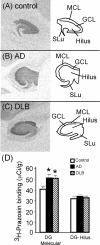
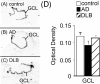
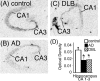
In Alzheimer's disease (AD), there is a significant loss of locus ceruleus (LC) noradrenergic neurons. However, functional and anatomical evidence indicates that the remaining noradrenergic neurons may be compensating for the loss. Because the noradrenergic system plays an important role in learning and memory, it is important to determine whether compensation occurs in noradrenergic neurons in the LC and hippocampus of subjects with AD or a related dementing disorder, dementia with Lewy bodies (DLB). We observed profound neuronal loss in the LC in AD and DLB subjects with three major changes in the noradrenergic system consistent with compensation: (1) an increase in tyrosine hydroxylase (TH) mRNA expression in the remaining neurons; (2) sprouting of dendrites into peri-LC dendritic zone, as determined by alpha2-adrenoreceptors (ARs) and norepinephrine transporter binding sites; and (3) sprouting of axonal projections to the hippocampus as determined by alpha2-ARs. In AD and DLB subjects, the postsynaptic alpha1-ARs were normal to elevated. Expression of alpha1A- and alpha2A-AR mRNA in the hippocampus of AD and DLB subjects were not altered, but expression of alpha1D- and alpha2C-AR mRNA was significantly reduced in the hippocampus of AD and DLB subjects. Therefore, in AD and DLB subjects, there is compensation occurring in the remaining noradrenergic neurons, but there does appear to be a loss of specific AR in the hippocampus. Because changes in these noradrenergic markers in AD versus DLB subjects were similar (except neuronal loss and the increase in TH mRNA were somewhat greater in DLB subjects), the presence of Lewy bodies in addition to plaques and tangles in DLB subjects does not appear to further affect the noradrenergic compensatory changes.
Figures
References
-
- Adolfsson R, Gottfries CG, Roos BE, Winblad B (1979) Changes in the brain catecholamines in patients with dementia of Alzheimer's type. Br J Psychiatry 135: 216–223. - PubMed
-
- Ballard C, Holmes C, McKeith I, Neill D, O'Brien J, Cairns N, Lantos P, Perry E, Ince P, Perry R (1999) Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer's disease. Am J Psychiatry 156: 1039–1045. - PubMed
-
- Barber R, Panikkar A, McKeith IG (2001) Dementia with Lewy bodies: diagnosis and management. Int J Geriatr Psychiatry 16: S12–S18. - PubMed
-
- Bauer ME, Tejani-Butt SM (1992) Effects of repeated administration of desipramine or electroconvulsive shock on norepinephrine uptake sites measured by [3H] nisoxetine autoradiography. Brain Res 582: 208–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
