Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods
- PMID: 16407545
- PMCID: PMC2597197
- DOI: 10.1523/JNEUROSCI.3915-05.2006
Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods
Abstract
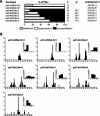
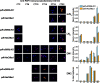
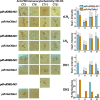
Coupling of autonomous cellular oscillators is an essential aspect of circadian clock function but little is known about its circuit requirements. Functional ablation of the pigment-dispersing factor-expressing lateral ventral subset (LNV) of Drosophila clock neurons abolishes circadian rhythms of locomotor activity. The hypothesis that LNVs synchronize oscillations in downstream clock neurons was tested by rendering the LNVs hyperexcitable via transgenic expression of a low activation threshold voltage-gated sodium channel. When the LNVs are made hyperexcitable, free-running behavioral rhythms decompose into multiple independent superimposed oscillations and the clock protein oscillations in the dorsal neuron 1 and 2 subgroups of clock neurons are phase-shifted. Thus, regulated electrical activity of the LNVs synchronize multiple oscillators in the fly circadian pacemaker circuit.
Figures





Similar articles
-
Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane-tethered spider toxin.PLoS Biol. 2008 Nov 4;6(11):e273. doi: 10.1371/journal.pbio.0060273. PLoS Biol. 2008. PMID: 18986214 Free PMC article.
-
Pigment dispersing factor-dependent and -independent circadian locomotor behavioral rhythms.J Neurosci. 2008 Jan 2;28(1):217-27. doi: 10.1523/JNEUROSCI.4087-07.2008. J Neurosci. 2008. PMID: 18171939 Free PMC article.
-
Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila.J Biol Rhythms. 2008 Apr;23(2):117-28. doi: 10.1177/0748730407312984. J Biol Rhythms. 2008. PMID: 18375861
-
Mechanisms of clock output in the Drosophila circadian pacemaker system.J Biol Rhythms. 2006 Dec;21(6):445-57. doi: 10.1177/0748730406293910. J Biol Rhythms. 2006. PMID: 17107935 Review.
-
The lateral and dorsal neurons of Drosophila melanogaster: new insights about their morphology and function.Cold Spring Harb Symp Quant Biol. 2007;72:517-25. doi: 10.1101/sqb.2007.72.063. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419311 Review.
Cited by
-
Allatostatin-A neurons inhibit feeding behavior in adult Drosophila.Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3967-72. doi: 10.1073/pnas.1200778109. Epub 2012 Feb 15. Proc Natl Acad Sci U S A. 2012. PMID: 22345563 Free PMC article.
-
Temperature synchronization of the Drosophila circadian clock protein PERIOD is controlled by the TRPA channel PYREXIA.Commun Biol. 2019 Jul 1;2:246. doi: 10.1038/s42003-019-0497-0. eCollection 2019. Commun Biol. 2019. PMID: 31286063 Free PMC article.
-
BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus.PLoS One. 2008;3(12):e3884. doi: 10.1371/journal.pone.0003884. Epub 2008 Dec 8. PLoS One. 2008. PMID: 19060951 Free PMC article.
-
Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane-tethered spider toxin.PLoS Biol. 2008 Nov 4;6(11):e273. doi: 10.1371/journal.pbio.0060273. PLoS Biol. 2008. PMID: 18986214 Free PMC article.
-
Potency of transgenic effectors for neurogenetic manipulation in Drosophila larvae.Genetics. 2015 Jan;199(1):25-37. doi: 10.1534/genetics.114.172023. Epub 2014 Oct 29. Genetics. 2015. PMID: 25359929 Free PMC article.
References
-
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH (2005) A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15: 886–893. - PubMed
-
- Blau J, Young MW (1999) Cycling vrille expression is required for a functional Drosophila clock. Cell 99: 661–671. - PubMed
-
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC (2004) Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429: 523–530. - PubMed
-
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
